Abstract
Dawson-type heteropolyoxometalate compounds (HPC) P2M18O62 -6 (M= Mo, W) have received increasing attention in the catalytic field due to the combination of redox and acidic properties in the same structure. It has been proved that the introduction of vanadium into the Keggin framework is beneficial for redox catalysis, shifting its activity from acid to redox-dominated.
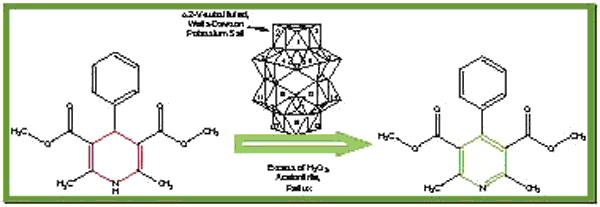
In this work we prepared and characterized vanadium (V) substituted Wells-Dawson heteropolysalt (WDKV). Fresh solid samples were characterized by 31P MAS-NMR, FTIR, SEM, XRD, TGA and Potentiometric titration measurements. Also, we performed the oxidation of a 1,4-dihydropyridine using WDKV as catalyst in acetonitrile media, with H2O2 as oxidant agent. The optimal procedure is the following: 1 mmol% of WDKV, a ratio 1,4-DHP: H2O2 (1:214) at reflux of acetonitrile. It must be noted that along all reactions, no secondary products were observed.
Keywords: Heteropolycompound, Oxidation, Hydrogen peroxide. 1, 4-dihydropyridine.
Current Catalysis
Title:Vanadium-Substituted Wells-Dawson Potassium Salt as Catalyst for Liquid phase Oxidation of 1,4-dihydropyridine Derivative
Volume: 3 Issue: 2
Author(s): Laura M. Sanchez, Angel G. Sathicq, Graciela T. Baronetti and Horacio J. Thomas
Affiliation:
Keywords: Heteropolycompound, Oxidation, Hydrogen peroxide. 1, 4-dihydropyridine.
Abstract: Dawson-type heteropolyoxometalate compounds (HPC) P2M18O62 -6 (M= Mo, W) have received increasing attention in the catalytic field due to the combination of redox and acidic properties in the same structure. It has been proved that the introduction of vanadium into the Keggin framework is beneficial for redox catalysis, shifting its activity from acid to redox-dominated.
In this work we prepared and characterized vanadium (V) substituted Wells-Dawson heteropolysalt (WDKV). Fresh solid samples were characterized by 31P MAS-NMR, FTIR, SEM, XRD, TGA and Potentiometric titration measurements. Also, we performed the oxidation of a 1,4-dihydropyridine using WDKV as catalyst in acetonitrile media, with H2O2 as oxidant agent. The optimal procedure is the following: 1 mmol% of WDKV, a ratio 1,4-DHP: H2O2 (1:214) at reflux of acetonitrile. It must be noted that along all reactions, no secondary products were observed.
Export Options
About this article
Cite this article as:
Sanchez M. Laura, Sathicq G. Angel, Baronetti T. Graciela and Thomas J. Horacio, Vanadium-Substituted Wells-Dawson Potassium Salt as Catalyst for Liquid phase Oxidation of 1,4-dihydropyridine Derivative, Current Catalysis 2014; 3 (2) . https://dx.doi.org/10.2174/2211544702666131224233041
| DOI https://dx.doi.org/10.2174/2211544702666131224233041 |
Print ISSN 2211-5447 |
| Publisher Name Bentham Science Publisher |
Online ISSN 2211-5455 |
 21
21
- Author Guidelines
- Graphical Abstracts
- Fabricating and Stating False Information
- Research Misconduct
- Post Publication Discussions and Corrections
- Publishing Ethics and Rectitude
- Increase Visibility of Your Article
- Archiving Policies
- Peer Review Workflow
- Order Your Article Before Print
- Promote Your Article
- Manuscript Transfer Facility
- Editorial Policies
- Allegations from Whistleblowers
- Announcements
Related Articles
-
Finding the Smoking Gun: Protein Tyrosine Phosphatases as Tools and Targets of Unicellular Microorganisms and Viruses
Current Medicinal Chemistry Human Exfoliated Deciduous Teeth Stem Cells: Features and Therapeutic Effects on Neurogenerative and Hepatobiliary-pancreatic Diseases
Current Stem Cell Research & Therapy Molecular, Cellular and Pharmaceutical Aspects of Biomaterials in Dentistry and Oral and Maxillofacial Surgery. An Internationalization of Higher Education and Research Perspective
Current Pharmaceutical Biotechnology Implications of Fibroblast Growth Factors (FGFs) in Cancer: From Prognostic to Therapeutic Applications
Current Drug Targets Pharmacotherapies to Manage Bone Loss-Associated Diseases: A Quest for the Perfect Benefit-to-Risk Ratio
Current Medicinal Chemistry Toll-like Receptor 4 Gene Polymorphisms in Chinese Population After Allogeneic Hematopoietic Stem Cell Transplantation
Current Bioinformatics Medicinal Use of Lincosamides and Microbial Resistance to Them
Anti-Infective Agents in Medicinal Chemistry Ellagic Acid Increases Osteocalcin and Alkaline Phosphatase After Tooth Extraction in Nicotinic-Treated Rats
Current Pharmaceutical Design Preparation, Physicochemical Evaluation and Antimicrobial Potential of Topical Dosage Forms Containing Natural Anti-inflammatory Agent, Curcuma longa
Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry In Situ Gelling Ophthalmic Drug Delivery System: An Overview and Its Applications
Recent Patents on Drug Delivery & Formulation Preparation, Physicochemical Evaluation and Characterization of Mucoadhesive Buccal Gels Impregnated with Benzydamine Hydrochloride for the Effective Treatment of Aphthous Stomatitis: Effect of Different Grades of HPMC Polymer on In vitro and Ex vivo Performance
Drug Delivery Letters Inflammation-Induced Thrombosis: Mechanisms, Disease Associations and Management
Current Pharmaceutical Design Contribution of Nanotechnology to Improved Treatment of Periodontal Disease
Current Pharmaceutical Design Bioscaffolds in Periodontal Regeneration
Nanoscience & Nanotechnology-Asia State of the Art of Biochemical Markers in Metastatic Bone Disease and the Role of Bisphosphonates as Therapeutic Agents
Current Pharmaceutical Analysis Patent Selections
Recent Patents on Endocrine, Metabolic & Immune Drug Discovery Meet Our Editorial Board Member
Recent Patents on Drug Delivery & Formulation Osteogenic Peptides in Bone Regeneration
Current Pharmaceutical Design Descending Necrotizing Mediastinitis of Odontogenic Origin
Recent Patents on Anti-Infective Drug Discovery Matrix Metalloproteinase (MMP)-7 Activates MMP-8 But Not MMP-13
Medicinal Chemistry