Abstract
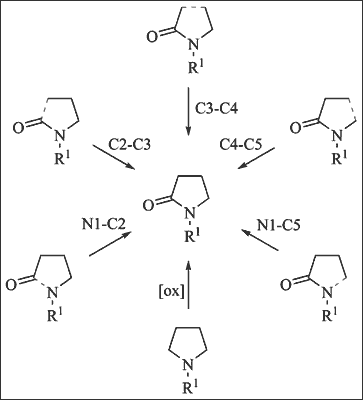
γ-Lactams (pyrrolidin-2-ones) and products containing the γ-lactam core are important synthetic targets and often display interesting biological activities, so that the development of improved synthetic methodologies for their preparation has become the objective of many synthetic chemists. This review is presented in two parts: Part I highlights efficient synthetic strategies (issued between 2001 and 2013), which are classified according to the last step of the lactam ring formation, starting from acyclic or cyclic precursors, whereas Part II deals with functionalization of γ-lactam ring via C-C or C-Heteroatom bond formation. Enantioselective synthesis is thoroughly emphasized in both sections, also exploiting catalytic systems, since pure enantiomeric derivatives are important for biological testing.
Keywords: Cyclization, enantioselectivity, lactams, stereoselectivity, synthetic methods.