Abstract
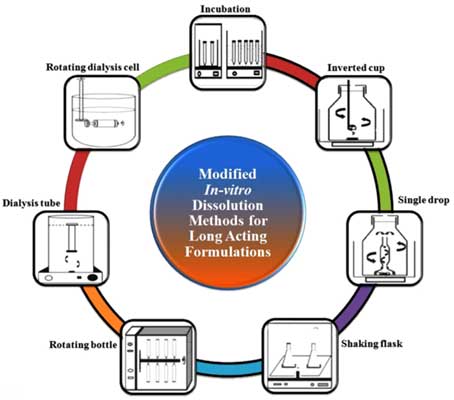
Long acting parenteral formulations are preferred over conventional formulations for the treatment of chronic diseases. Prevalence of such diseases provoked the interest of researchers and pharmaceutical industries in the development of long acting parenteral formulations. The regulatory guidelines and pharmacopoeia have remained silent on dissolution methods for long acting parenteral formulations due to their diverse nature. The lack of compendial method for dissolution testing increases the duration of approval process for long acting parenteral formulations. This article reviews various dissolution methods used to study in vitro drug release profile of long acting parenteral formulations. Compendial as well as noncompendial methods, such as- rotating dialysis cell, dialysis tube, rotating bottle, shaking flask, single drop, inverted cup and incubation, are used by researchers for drug release profile of long acting parenteral formulations. This review article also highlights the advantages and disadvantages of reported dissolution methods along with the suitability of these methods for particular type of long acting formulation. The compiled work will help the researchers in designing the biorelevant dissolution method and expedite the development of long acting parenteral formulations.
Keywords: Dissolution apparatus, injectable, in vitro models, long acting formulations, parenteral.
Current Drug Delivery
Title:Current Approaches for in vitro Drug Release Study of Long Acting Parenteral Formulations
Volume: 12 Issue: 3
Author(s): Tejas M. Dadhaniya, Om Prakash Sharma, Mukesh C. Gohel and Priti J. Mehta
Affiliation:
Keywords: Dissolution apparatus, injectable, in vitro models, long acting formulations, parenteral.
Abstract: Long acting parenteral formulations are preferred over conventional formulations for the treatment of chronic diseases. Prevalence of such diseases provoked the interest of researchers and pharmaceutical industries in the development of long acting parenteral formulations. The regulatory guidelines and pharmacopoeia have remained silent on dissolution methods for long acting parenteral formulations due to their diverse nature. The lack of compendial method for dissolution testing increases the duration of approval process for long acting parenteral formulations. This article reviews various dissolution methods used to study in vitro drug release profile of long acting parenteral formulations. Compendial as well as noncompendial methods, such as- rotating dialysis cell, dialysis tube, rotating bottle, shaking flask, single drop, inverted cup and incubation, are used by researchers for drug release profile of long acting parenteral formulations. This review article also highlights the advantages and disadvantages of reported dissolution methods along with the suitability of these methods for particular type of long acting formulation. The compiled work will help the researchers in designing the biorelevant dissolution method and expedite the development of long acting parenteral formulations.
Export Options
About this article
Cite this article as:
Dadhaniya M. Tejas, Sharma Prakash Om, Gohel C. Mukesh and Mehta J. Priti, Current Approaches for in vitro Drug Release Study of Long Acting Parenteral Formulations, Current Drug Delivery 2015; 12 (3) . https://dx.doi.org/10.2174/1567201812666150209143731
| DOI https://dx.doi.org/10.2174/1567201812666150209143731 |
Print ISSN 1567-2018 |
| Publisher Name Bentham Science Publisher |
Online ISSN 1875-5704 |
Call for Papers in Thematic Issues
Advances of natural products, bio-actives and novel drug delivery system against emerging viral infections
Due to the increasing prevalence of viral infections and the ability of these human pathogens to develop resistance to current treatment strategies, there is a great need to find and develop new compounds to combat them. These molecules must have low toxicity, specific activity and high bioavailability. The most suitable ...read more
Electrospun Fibers as Drug Delivery Systems
In recent years, electrospun fibers have attracted considerable attention as potential platforms for drug delivery due to their distinctive properties and adaptability. These fibers feature a notable surface area-to-volume ratio and can be intentionally designed with high porosity, facilitating an increased capacity for drug loading and rendering them suitable for ...read more
Emerging Nanotherapeutics for Mitigation of Neurodegenerative Disorders
Conditions affecting the central nervous system (CNS) present a significant hurdle due to limited access of both treatments and diagnostic tools for the brain. The blood-brain barrier (BBB) acts as a barrier, restricting the passage of molecules from the bloodstream into the brain. The most formidable challenge facing scientists is ...read more
Nanotechnology Based Chemotherapy for the treatment of Head & Neck Cancer
The escalating recurrence rates observed in Head and Neck cancer, particularly within the chemo-therapeutically treated cohort (50-60%), can be attributed to the non-selective nature of current anticancer drug delivery modalities. In this context, nanotechnology-based drug delivery systems emerge as a promising avenue for achieving precise localization of therapeutic agents to ...read more
 137
137 9
9
- Author Guidelines
- Graphical Abstracts
- Fabricating and Stating False Information
- Research Misconduct
- Post Publication Discussions and Corrections
- Publishing Ethics and Rectitude
- Increase Visibility of Your Article
- Archiving Policies
- Peer Review Workflow
- Order Your Article Before Print
- Promote Your Article
- Manuscript Transfer Facility
- Editorial Policies
- Allegations from Whistleblowers
Related Articles
-
Hormones and the Autonomic Nervous System are Involved in Suprachiasmatic Nucleus Modulation of Glucose Homeostasis
Current Diabetes Reviews From the Design to the Clinical Application of Thromboxane Modulators
Current Pharmaceutical Design Modular Nanotransporters for Targeted Intracellular Delivery of Drugs: Folate Receptors as Potential Targets
Current Pharmaceutical Design Growth of Diabetes Research in United Arab Emirates: Current and Future Perspectives
Current Diabetes Reviews Current Status on Immunoprotection of Transplanted Islets: Focus on Islet Microencapsulation
Micro and Nanosystems Anemia in Patients with Diabetic Foot Ulcer: Effects on Diabetic Microvascular Complications and Related Conditions
Endocrine, Metabolic & Immune Disorders - Drug Targets Alkaloids and Flavonoids as α1-Adrenergic Receptor Antagonists
Current Medicinal Chemistry Identification of Novel Key Targets and Candidate Drugs in Oral Squamous Cell Carcinoma
Current Bioinformatics Metabotropic Glutamate Receptors and Interacting Proteins: Evolving Drug Targets
Current Drug Targets Insight into the Epigenetics of Alzheimer's Disease: A Computational Study from Human Interactome
Current Alzheimer Research Low 25 Hydroxyvitamin D Levels are Independently Associated with Autoimmune Thyroiditis in a Cohort of Apparently Healthy Overweight and Obese Subjects
Endocrine, Metabolic & Immune Disorders - Drug Targets Gene Variants and Haplotypes Modifying Transcription Factor Binding Sites in the Human Cyclooxygenase 1 and 2 (PTGS1 and PTGS2) Genes
Current Drug Metabolism High-Density Lipoprotein and Low-Density Lipoprotein Subfractions in Patients with Chronic Kidney Disease
Current Vascular Pharmacology Meet Our Editorial Board Member
Current Drug Targets Therapeutic Challenges in Neuroendocrine Tumors
Anti-Cancer Agents in Medicinal Chemistry The Renin-Angiotensin System and Advanced Glycation End-Products in Diabetic Retinopathy: Impacts and Synergies.
Current Clinical Pharmacology Cancer Therapy By Targeting Hypoxia-Inducible Factor-1
Current Cancer Drug Targets GPCRs and Insulin Receptor Signaling in Conversation: Novel Avenues for Drug Discovery
Current Topics in Medicinal Chemistry The Application of Prodrug-based Drug Delivery Strategy in Anticancer Drugs
Current Topics in Medicinal Chemistry Current Therapies and Emerging Targets for the Treatment of Diabetes
Current Pharmaceutical Design