Abstract
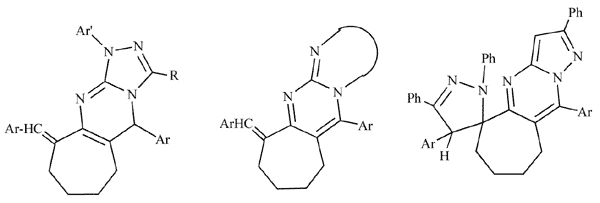
A series of novel cyclohepta[1,2-d][1,2,4]triazolo[4,3-a]pyrimidines was prepared by reaction of 9- arylmethylene-cycloheptapyrimidine-2-thione with hydrazonoyl halides in dioxane in the presence of triethylamine. Also, a series of fused cycloheptapyrimidines was synthesized via reaction of 2,7-diarylmethylenecycloheptanone with heterocyclic amines. The products 14a-c of the latter reaction were used as starting materials since they contain an olefinic exocyclic C=C and endocyclic C=N bonds. 1,3-Dipolar cycloaddition reaction of these products with hydrazonoyl halides in benzene in the presence of triethylamine afforded novel spiropyrazolines. All the above reactions proceeded site and regio-selectively, and the structures of the products were established based on both elemental and spectral analysis data (IR, 1H NMR, MS). In addition, the biological activity of some of the new products was evaluated and the results obtained revealed medium to high activity against some bacteria and fungi species.
Keywords: 2, 7-Diarylmethylene-cycloheptanone, hydrazonoyl halides, site-selectivity, spiropyrazolines, antimicrobial activity.