Abstract
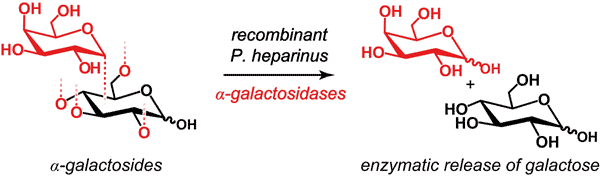
Two putative α-D-galactosidases (α-GALs) belonging to glycosyl hydrolase family 27, and originating from the rather unexplored bacterial strain Pedobacter heparinus, were cloned and biochemically characterized. The recombinant enzymes designated as PhAGal729 and PhAGal2920 showed comparable biochemical properties: the optimum pH values were determined to be pH 5.0 and 5.5, and temperature optima lay between 30°C and 37°C, respectively. Both α-GALs were not dependent on the presence of divalent metal ions, and the addition of EDTA had no influence on enzymatic activity. The activity of both enzymes substantially increased in the presence of Fe3+ ions. Both enzymes were inhibited by sodium dodecyl sulfate (SDS) and urea. α-GALs from P. heparinus were highly specific in hydrolyzing glycosides with α-1,2/3/4 or α-1,6-linked galactose to other sugars, whereas other glycosides such as α-linked N-acetylgalactosamine, N-acetylglucosamine or glucose residues were not released. Nevertheless, neither PhAGal729 nor PhAGal2920 were able to remove α-linked galactose epitopes from native human erythrocytes. The facile expression and purification procedures in combination with wide substrate specificities make α-GALs from P. heparinus potential candidates for applications in analytical research, and food- and biotechnology.
Keywords: Galactosidase, galactoside, , Pedobacter heparinus, bacterial glycosidase, glycoenzyme.