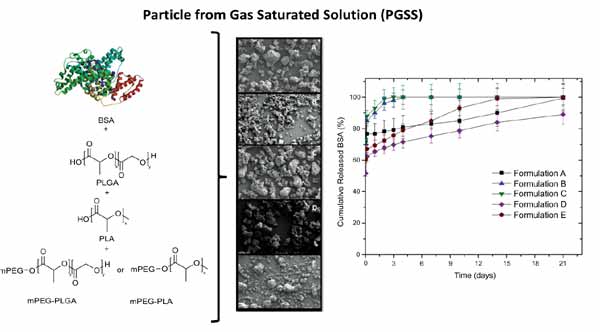
Abstract
Background: Particles from Gas Saturated Solution (PGSS) is an emergent method that employs supercritical carbon dioxide (scCO2) to produce microparticles. It is suitable for encapsulating biologically active compounds including therapeutic peptides and proteins. Poly(lactide acid) (PLA) and/or poly(lactic-coglycolic acid) (PLGA) are the most commonly used materials in PGSS, due to their good processability in scCO2. Previous studies demonstrated that the properties of the microparticles can be modulated by adding polyethylene glycol (PEG) or tri-block PEGylated copolymers.
Objective: In the present work, the effect of the addition of biodegradable PEGylated di-block copolymers on the physical properties and drug release performance of microparticles prepared by PGSS technique was evaluated.
Method: mPEG5kDa-P(L)LA and mPEG5kDa-P(L)LGA with similar molecular weights were synthesized and their behaviour, when exposed to supercritical CO2, was investigated. Different microparticle formulations, composed of a high (81%) or low (9%) percentage of the synthesized copolymers were prepared and compared in terms of particle size distribution, morphology, yield and protein release. Drug release studies were performed using bovine serum albumin (BSA) as a model protein.
Results: PEGylated copolymers showed good processability in PGSS without significant changes to the physical properties of the microparticles. However, the addition of PEG exerted a modulating effect on the microparticle drug dissolution behaviour, increasing the rate of BSA release as a function of its content in the formulation.
Conclusion: This study demonstrated the feasibility of producing microparticles by using PEGylated di-block copolymers through a PGSS technique at mild operating conditions (low operating pressure and temperature).
Keywords: BSA, Di-block copolymers, drug release, differential scanning calorimetry (DSC), microparticles, view cell.