Abstract
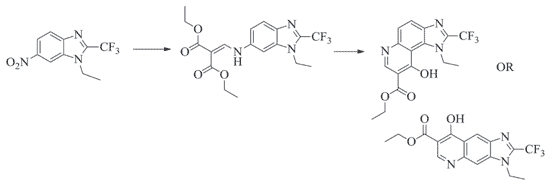
A series of novel trifluoromethyl substituted imidazo fused quinolone analogues were prepared, isolated and characterized. The title compounds, thus ob-tained, were synthesized from compound 3. Initially, the nitro group of compound 3 was reduced using Pd/C and hydrazine hydrate afforded the compound 5. The amine 5 was treated with EMME furnished the corresponding imidazo fused quinolines as angular compound 7. The alkylation reaction of 7 with different alkyl halides furnished the corresponding N-alkylated derivatives 9a-h. N-alkylated fluoro quinolones on hydrolysis using 10% LiOH furnished the corresponding carboxylic acid derivatives 10a-h in good yields. All the newly synthesized trifluoromethyl substituted imidazo fused quinolone analogues were further evaluated for their antibacteri-al activity against the four bacterial strains. Among them, two were gram positive i.e., Staphylococcus aureus and Bacillus subtilis bacteria and two were gram negative bacteria i.e., Klebsiella pneumonia and E. coli. Some of the compounds such as 7, 9a, 9d, 9f, 10f and 10g showed good antibacterial activi-ty against all bacterial strains when compared to standard drugs.
Keywords: Trifluoromethyl, benzimidazole, imidazole, quinolones, antibacterial activity, docking studies.