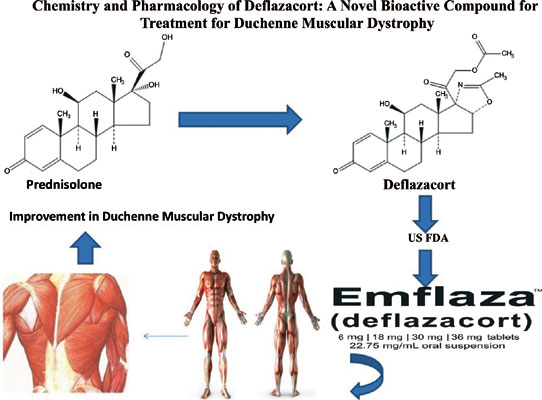
[1]
Mendell, J.R.; Shilling, C.; Leslie, N.D.; Flanigan, K.M.; al-Dahhak, R.; Gastier-Foster, J.; Kneile, K.; Dunn, D.M.; Duval, B.; Aoyagi, A.; Hamil, C.; Mahmoud, M.; Roush, K.; Bird, L.; Rankin, C.; Lilly, H.; Street, N.; Chandrasekar, R.; Weiss, R.B. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann. Neurol., 2012, 71(3), 304-313.
[2]
Ghosh, T.; Ghosh, U.; Ghosh, G.; Malik, D.; Bose, S.; Basu, U. Developing effective therapy for duchenne muscular dystrophy (DMD). Challenges and Promises. Curr. Chemical Biol., 2014, 8(3), 117-131.
[3]
Dooley, J.; Gordon, K.E.; Dodds, L.; MacSween, J. Duchenne muscular dystrophy: a 30-year population-based incidence study. Clin. Pediatr. (Phila.), 2010, 49(2), 177-179.
[4]
Ciafaloni, E.; Fox, D.J.; Pandya, S.; Westfield, C.P.; Puzhankara, S.; Romitti, P.A.; Mathews, K.D.; Miller, T.M.; Matthews, D.J.; Miller, L.A.; Cunniff, C.; Druschel, C.M.; Moxley, R.T. Delayed diagnosis in duchenne muscular dystrophy: data from the muscular dystrophy surveillance, tracking, and research network (MD STARnet). J. Pediatr., 2009, 155(3), 380-385.
[5]
Bushby, K.M.; Hill, A.; Steele, J.G. Failure of early diagnosis in symptomatic Duchenne muscular dystrophy. Lancet, 1999, 353(9152), 557-558.
[6]
Ervasti, J.M.; Ohlendieck, K.; Kahl, S.D.; Gaver, M.G.; Campbell, K.P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature, 1990, 345(6273), 315-319.
[7]
Zubrzycka-Gaarn, E.E.; Bulman, D.E.; Karpati, G.; Burghes, A.H.; Belfall, B.; Klamut, H.J.; Talbot, J.; Hodges, R.S.; Ray, P.N.; Worton, R.G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature, 1988, 333(6172), 466-469.
[8]
Ameen, V.; Robson, L.G. Experimental models of duchenne muscular dystrophy: relationship with cardiovascular disease. Open Cardiovasc. Med. J., 2010, 4, 265-277.
[9]
Nayak, S.; Acharjya, B. Deflazacort versus other glucocorticoids: a comparison. Indian J. Dermatol., 2008, 53(4), 167-170.
[10]
Markham, A.; Bryson, H.M. Deflazacort. A review of its pharmacological properties and therapeutic efficacy. Drugs, 1995, 50(2), 317-333.
[11]
Ambalal, P.S.; Natvarlal, J.P. Validated spectrophotometric methods for determination of deflazacort in tablet dosage form. Asian J. Pharm. Life Sci., 2011, 1(3), 200-207.
[13]
Selvadurai, M.; Meyyanathan, S.N. Determination of deflazacort in human plasma by liquid chromatography-mass spectrometry after liquid-liquid extraction and its application in human pharmacokinetics studies. Pharm. Methods, 2011, 2(2), 106-111.
[14]
Sharma, Y.; Jain, N.; Jain, H.; Jain, P.K.; Jain, A.K. Development and validation of RP-HPLC method of deflazacort for stability indicating assay. Int. J. Pharm., 2014, 4(2), 117-123.
[15]
Scremin, A.; Piazzon, M.; Silva, M.A.S.; Kuminek, G.; Correa, G.M.; Paulino, N.; Cardoso, C.G. Spectrophotometric and HPLC determination of deflazacort in pharmaceutical dosage forms. Braz. J. Pharm. Sci., 2010, 46(2), 281-287.
[16]
Luzzani, F.; Glässer, A. Differential binding in vitro to glucocorticoid receptors of deflazacort and prednisolone. Eur. J. Pharmacol., 1981, 76(4), 427-430.
[17]
Assandri, A.; Buniva, G.; Martinelli, E.; Perazzi, A.; Zerilli, L. Pharmacokinetics and metabolism of deflazacort in the rat, dog, monkey and man. Adv. Exp. Med. Biol., 1984, 171, 9-23.
[18]
Robert, C.G.; Miller, J.P.; Greenberg, C.R. Duchenne muscular dystrophy. Neurology, 2016, 87(20), 2123-2131.
[20]
Martinelli, E.; Ferrari, P.; Ripamonti, A.; Tuan, G.; Perazzi, A.; Assandri, A. Metabolism of deflazacort in the rat, dog and man. Drug Metab. Dispos., 1979, 7(5), 335-339.