Abstract
Background: Resveratrol is a wonder therapy for the treatment of several skin disorders, including psoriasis, but its skin permeation limits its applications.
Objective: The present work dealt with optimizing and formulating resveratrol loaded vitamin E based nanoemulsion and carbomer based nanoemulgel intended for topical application in the treatment of plaque psoriasis.
The major objective of this study was to achieve the quality target product profile with respect to enhanced skin permeation and superior skin deposition of the formulated nanoemulgel to achieve the superlative therapeutic advantages.
Methods: Formulation by design (FbD) approach was employed to optimize varied critical material attributes such as the concentration of oil and Smix to achieve the desired quality characteristics. Carbomer based nanoemulgel was formulated and evaluated.
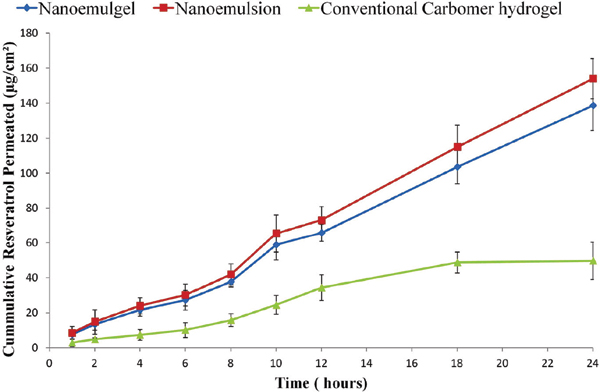
Results: Optimized formulation was having globule size (168.3 ± 4.98 nm), percentage cumulative permeation (4.81 ± 0.65%), permeation flux (7.62 ± 0.39 μg hr-1cm-2), and skin deposition (668.65 ± 11.98 μg cm-2). Nanoemulgel was found to have optimum physical properties in terms of viscosity, spreadability, pH and physical stability. The extent of skin deposition was approximately 6.682 times higher while the permeation enhancement ratio was around 2.872 as compared to conventional formulation indicating its higher skin targeting abilities, which was further ratified by Confocal Laser Scanning Microscopy results.
Conclusion: Nanoemulgel formulated by the current FbD approach has enhanced skin permeation and skin deposition properties as compared to conventional carbomer gel. Thus, it could augment the therapeutic benefits of encapsulated bioactive in the treatment of several skin disorders like psoriasis.
Keywords: Factorial design, psoriasis, resveratrol, skin permeation, skin targeting, vitamin E nanoemulgel.
[http://dx.doi.org/10.3109/10837450.2015.1026605] [PMID: 26024238]
[http://dx.doi.org/10.1016/j.ijbiomac.2018.08.136] [PMID: 30171962]
[http://dx.doi.org/10.22270/jddt.v8i5-s.1914]
[http://dx.doi.org/10.1615/CritRevTherDrugCarrierSyst.v22.i1.20] [PMID: 15715503]
[http://dx.doi.org/10.1016/j.ijpharm.2016.09.014] [PMID: 27609664]
[http://dx.doi.org/10.1039/C7FO01086A] [PMID: 29034918]
[http://dx.doi.org/10.1615/CritRevTherDrugCarrierSyst.v22.i3.10] [PMID: 15896189]
[http://dx.doi.org/10.1088/0957-4484/25/48/485102] [PMID: 25392203]
[http://dx.doi.org/10.1080/03639045.2019.1648500] [PMID: 31353967]
[http://dx.doi.org/10.1080/03639045.2017.1391836] [PMID: 29096550]
[http://dx.doi.org/10.1007/s00403-019-01964-3] [PMID: 31432208]
[http://dx.doi.org/10.7324/JAPS.2015.50704]
[http://dx.doi.org/10.1016/j.ijpharm.2019.118448] [PMID: 31226472]
[http://dx.doi.org/10.3109/02652048.2015.1046513] [PMID: 26066775]
[http://dx.doi.org/10.2174/156652310791111010] [PMID: 20353386]
[http://dx.doi.org/10.2174/1573401314666180223134235]
[http://dx.doi.org/10.5530/jyp.2018.2s.20]
[http://dx.doi.org/10.1080/1061186X.2017.1379527] [PMID: 28895754]
[http://dx.doi.org/10.1016/j.ejpb.2017.04.008] [PMID: 28411056]
[http://dx.doi.org/10.1080/10717544.2016.1223225] [PMID: 27689408]
[http://dx.doi.org/10.3109/10717544.2014.920432] [PMID: 24865289]
[http://dx.doi.org/10.3109/1061186X.2015.1077845] [PMID: 26302815]