Abstract
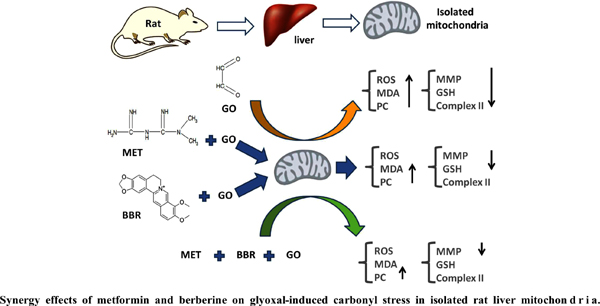
Objective: Carbonyl stress, resulting from toxic effects of alpha-dicarbonyls such as glyoxal (GO), plays an important role in mitochondrial dysfunction and subsequent development of diabetic complications. This study evaluated the ability of metformin (MET), berberine (BBR), and their combination to prevent GO-induced carbonyl stress in isolated rat liver mitochondria.
Methods: Mitochondria (0.5 mg protein/mL) were isolated from the Wistar rat liver and incubated with various concentrations of GO (1, 2.5, 5, 7.5, and 10 mM) for 30 minutes and IC50 for GO was calculated. The suspensions of mitochondria were incubated with various concentrations of MET (2.5, 5, 10, and 20 mM) or BBR (2.5, 5, 10, and 20 μM) for 30 min and then GO in a dose of IC50 at 37 ºC for 30 min. Mitochondrial complex II activity, mitochondrial membrane potential (MMP), MDA level, reactive oxygen species (ROS) formation, reduced glutathione (GSH) content, and protein carbonylation were assessed. The combination index and isobologram of MET and BBR on GO toxicity were calculated.
Results: IC50 of GO was assigned approximately 3 mM. GO disrupted the electron transfer chain and significantly increased mitochondrial ROS formation, protein carbonylation, and MDA level. GO decreased mitochondrial viability, MMP, and GSH content. Pre-treatment with MET and BBR could potentially reverse GO-induced deleterious effects in a concentration-dependent manner. Results of the drug combination indicated that CI for Fa 0.5 (Effect 50 %) was 0.83.
Conclusion: These results suggest that BBR in combination with MET has a moderate synergistic effect on GO-induced carbonyl stress in isolated rat liver mitochondria.
Keywords: Glyoxal, carbonyl stress, metformin, berberine, isobologram, mitochondria.
[http://dx.doi.org/10.1016/j.diabres.2017.03.024] [PMID: 28437734]
[http://dx.doi.org/10.4103/2230-8210.183480] [PMID: 27366724]
[http://dx.doi.org/10.3748/wjg.15.4137] [PMID: 19725147]
[http://dx.doi.org/10.3390/ijms18050984] [PMID: 28475116]
[http://dx.doi.org/10.1177/0960327117715900] [PMID: 28639457]
[http://dx.doi.org/10.2337/diabetes.53.2007.S110] [PMID: 14749275]
[http://dx.doi.org/10.2337/diaclin.21.4.175]
[http://dx.doi.org/10.1053/meta.2003.50093] [PMID: 12759888]
[http://dx.doi.org/10.1016/j.metabol.2005.01.029] [PMID: 15931622]
[http://dx.doi.org/10.1016/j.cbi.2008.10.026] [PMID: 19010314]
[http://dx.doi.org/10.3390/molecules21030280] [PMID: 26927058]
[http://dx.doi.org/10.1016/j.fitote.2013.11.010] [PMID: 24321576]
[http://dx.doi.org/10.1016/j.atherosclerosis.2015.09.032] [PMID: 26520899]
[http://dx.doi.org/10.1016/j.mce.2017.01.009] [PMID: 28087385]
[http://dx.doi.org/10.1016/j.ejphar.2010.12.030] [PMID: 21236251]
[http://dx.doi.org/10.1016/0003-2697(76)90527-3] [PMID: 942051]
[http://dx.doi.org/10.1016/j.jinorgbio.2009.11.007] [PMID: 20015552]
[http://dx.doi.org/10.1016/j.etap.2008.04.003] [PMID: 21783917]
[PMID: 24250581]
[http://dx.doi.org/10.1016/S0005-2728(03)00110-5] [PMID: 14507434]
[http://dx.doi.org/10.1016/j.cbi.2008.10.003] [PMID: 18983988]
[http://dx.doi.org/10.1016/S0009-8981(03)00003-2] [PMID: 12589963]
[http://dx.doi.org/10.1007/s11010-007-9535-1] [PMID: 17594057]
[http://dx.doi.org/10.1186/1479-5876-11-24] [PMID: 23360542]
[http://dx.doi.org/10.1016/j.bbadis.2011.10.008] [PMID: 22027215]
[http://dx.doi.org/10.1016/j.lfs.2012.08.017] [PMID: 22925597]
[http://dx.doi.org/10.1016/j.apsb.2012.06.003]
[http://dx.doi.org/10.1139/Y09-136] [PMID: 20393601]
[http://dx.doi.org/10.1161/CIRCULATIONAHA.112.132159] [PMID: 23564668]
[http://dx.doi.org/10.1179/174329211X13049558293713] [PMID: 22195989]
[http://dx.doi.org/10.1038/srep23664] [PMID: 27173483]
[http://dx.doi.org/10.1152/ajpendo.2001.280.5.E685] [PMID: 11287350]
[http://dx.doi.org/10.1089/rej.2016.1826] [PMID: 27185159]
[http://dx.doi.org/10.1089/rej.2016.1883] [PMID: 27897089]
[http://dx.doi.org/10.1016/j.pharep.2013.11.008] [PMID: 24905518]
[PMID: 24250680]
[http://dx.doi.org/10.1002/jcb.25907] [PMID: 28169456]
[http://dx.doi.org/10.1016/j.bbabio.2013.01.004] [PMID: 23333272]
[http://dx.doi.org/10.1016/j.bbabio.2012.12.007] [PMID: 23291191]
[http://dx.doi.org/10.1016/S0014-5793(00)01082-6] [PMID: 10682852]
[http://dx.doi.org/10.1038/s41598-017-08308-z] [PMID: 28811603]
[PMID: 27097449]
[http://dx.doi.org/10.3109/0886022X.2016.1165120] [PMID: 27056079]
[PMID: 26550442]
[http://dx.doi.org/10.1038/ki.2010.11] [PMID: 20164825]
[http://dx.doi.org/10.3390/ijms13067694] [PMID: 22837722]