Abstract
Background: Inflammation is a multifactorial process reflecting the response of the organism to various stimuli and is associated with a number of disorders such as arthritis, asthma and psoriasis, which require long-lasting or repeated treatment.
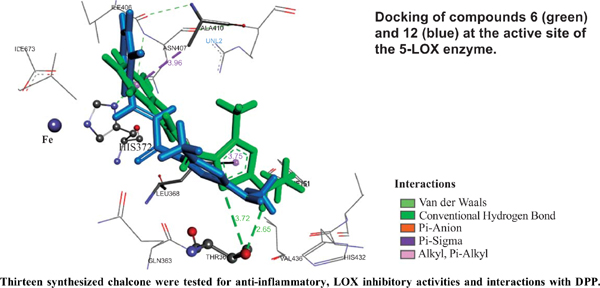
Objective: The aim of this paper is to evaluate the anti-inflammatory activity of previous synthesized thiazole-based chalcone derivatives.
Methods: Chalcones were synthesized via Cliazen-Schmidt condensation1-(4-methyl-2- alkylamino)thiazol-5-yl) ethanone with a corresponding aromatic aldehyde. For the evaluation of possible anti-inflammatory activity, carrageenan mouse paw edema was used.
Results: Eight out of thirteen tested chalcones showed anti-inflammatory activity in a range of 51- 55%. Prediction of toxicity revealed that these compounds are not toxic.
Conclusion: In general, it can be concluded that these compounds can be used for further modifications in order to develop more active and safe agents.
Keywords: Thiazole, Chalcone, NSAIDs, Toxicity, Docking, LOX.
[http://dx.doi.org/10.1016/0090-6980(81)90036-8] [PMID: 6270742]
[http://dx.doi.org/10.1016/j.ejmech.2008.04.006] [PMID: 18534720]
[http://dx.doi.org/10.1021/jm701496h] [PMID: 18311898]
[http://dx.doi.org/10.1016/j.ejmech.2008.05.029] [PMID: 18603333]
[http://dx.doi.org/10.1016/j.bmc.2012.10.046] [PMID: 23219856]
[http://dx.doi.org/10.3109/14756366.2011.636362] [PMID: 22380777]
[http://dx.doi.org/10.1007/s00044-017-1963-1]
[http://dx.doi.org/10.1016/j.ejmech.2015.08.005] [PMID: 26291036]
[http://dx.doi.org/10.3390/molecules24030539] [PMID: 30717217]
[http://dx.doi.org/10.1007/s00044-017-1851-8]
[http://dx.doi.org/10.1358/mf.2006.28.7.1003549] [PMID: 17003846]
[http://dx.doi.org/10.4269/ajtmh.1982.31.1164] [PMID: 7149102]
[http://dx.doi.org/10.1080/14756366.2016.1265517] [PMID: 28118738]
[http://dx.doi.org/10.1038/s41429-020-0280-y] [PMID: 31988484]
[http://dx.doi.org/10.1016/j.bioorg.2019.03.033] [PMID: 30921740]
[http://dx.doi.org/10.2174/1568026617666170914160446] [PMID: 28914193]
[http://dx.doi.org/10.3109/14756366.2014.949255] [PMID: 25198887]
[http://dx.doi.org/10.1248/bpb.b14-00099] [PMID: 24989010]
[http://dx.doi.org/10.3390/molecules24183259] [PMID: 31500191]
[http://dx.doi.org/10.1186/s12936-019-3060-z] [PMID: 31842914]
[http://dx.doi.org/10.1016/j.ejmech.2018.04.057] [PMID: 29758517]
[http://dx.doi.org/10.1016/j.ejmech.2018.03.047] [PMID: 29602038]
[http://dx.doi.org/10.3390/molecules25051047] [PMID: 32110945]
[http://dx.doi.org/10.1016/j.apsb.2019.01.003] [PMID: 30972281]
[http://dx.doi.org/10.1016/j.bmcl.2018.04.042] [PMID: 29724588]
[http://dx.doi.org/10.1007/s00044-018-2183-z]
[http://dx.doi.org/10.1016/j.ejmech.2014.10.068] [PMID: 25462272]
[http://dx.doi.org/10.2174/0929867325666181001112226] [PMID: 30277140]
[http://dx.doi.org/10.1016/j.bmcl.2004.01.021] [PMID: 15006393]
[http://dx.doi.org/10.2174/1568026619666190129121933] [PMID: 30706816]
[http://dx.doi.org/10.1016/S0023-6438(95)80008-5]
[PMID: 19499576]
[http://dx.doi.org/10.1126/science.1197203] [PMID: 21233389]
[http://dx.doi.org/10.1038/s41589-020-0544-7] [PMID: 32393899]
[http://dx.doi.org/10.3390/molecules23030685] [PMID: 29562646]
[http://dx.doi.org/10.3390/antiox8040090] [PMID: 30959820]
[http://dx.doi.org/10.1016/j.ejmech.2010.01.060] [PMID: 20153559]
[http://dx.doi.org/10.1016/j.bmc.2008.02.079] [PMID: 18343123]
[http://dx.doi.org/10.1016/j.str.2012.06.003] [PMID: 22795085]