Abstract
Background: Bronchial Asthma (BA) and Chronic Obstructive Pulmonary Disease (COPD) are chronic airway inflammation diseases. In recent years, patients with signs of both BA and COPD have been assigned to a separate group as Asthma-COPD Overlap Syndrome (ACOS). Free-circulating plasma microRNAs are considered as potential biomarkers of pulmonology diseases, including BA, COPD, and ACOS.
Objective: This study aimed to investigate the expression level of free-circulating plasma microRNAs, hsa-miR-19b-3p, hsa-miR-125b-5p, and hsa-miR-320c in patients with BA, COPD and ACOS for the detection and validation of new microRNAs as biomarkers for chronic lung diseases.
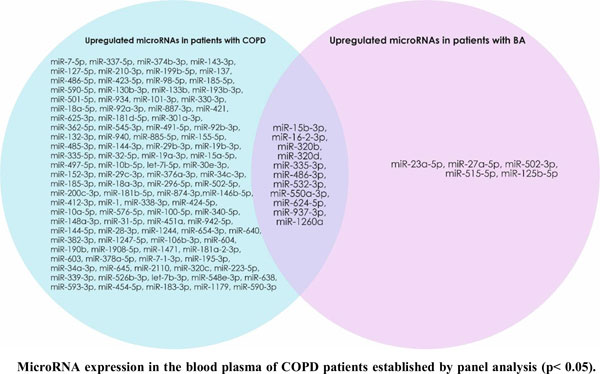
Methods: The relative expression levels of 720 microRNAs were evaluated by Real Time-Polymerase Chain Reaction (RT-PCR) in patients with COPD and BA. Three upregulated microRNAs (hsa-miR-19b-3p, hsa-miR-125b-5p and hsa-miR-320c) were selected for further study. The obtained data were analyzed using the microRNA PCR Array Data Analysis tool. The sensitivity and specificity were estimated using the area under the Receiver Operating Characteristics curve (ROC).
Results: The expression level of free-circulating hsa-miR-19b-3p was decreased in the blood plasma of patients with BA and ACOS, and increased in patients with COPD. hsa-miR-125b-5p was downregulated in the blood plasma of patients with COPD and upregulated in patients with BA and ACOS. hsa-miR-320c was downregulated in the blood plasma of patients with BA, and upregulated in patients with COPD and ACOS. The ROC curves of patients with BA for hsa-miR-19b-3p, patients with ACOS for hsa-miR-125b-5p, and patients with COPD for hsa-miR-320c revealed the probability of them as valuable biomarkers with AUCs of 0.824, 0.825, and 0.855, respectively.
Conclusion: Our study revealed three promising biomarkers for the diagnosis of COPD, BA and ACOS.
Keywords: Circulating hsa-miR-19b-3p, hsa-miR-320c, hsa-miR-125b-5p, bronchial asthma, chronic obstructive pulmonarydisease, Asthma-COPD Overlap Syndrome (ACOS).
[http://dx.doi.org/10.1016/j.alit.2018.01.002] [PMID: 29433946]
[PMID: 26251584]
[http://dx.doi.org/10.1186/1465-9921-14-112] [PMID: 24143997]
[http://dx.doi.org/10.1155/2016/7802521] [PMID: 27376086]
[http://dx.doi.org/10.2147/COPD.S130616] [PMID: 28694694]
[http://dx.doi.org/10.3390/ijms20030553] [PMID: 30696075]
[http://dx.doi.org/10.1016/j.intimp.2018.12.013] [PMID: 30578969]
[http://dx.doi.org/10.1515/hsz-2017-0249] [PMID: 29148977]
[http://dx.doi.org/10.2174/2211536605666161027165915] [PMID: 27804865]
[http://dx.doi.org/10.1186/s12943-018-0766-4] [PMID: 29415727]
[http://dx.doi.org/10.1016/j.tcb.2006.12.007] [PMID: 17197185]
[http://dx.doi.org/10.1016/j.canlet.2014.10.011] [PMID: 25451319]
[http://dx.doi.org/10.1080/08820139.2019.1578230] [PMID: 31012336]
[http://dx.doi.org/10.1186/1939-4551-4-6-94] [PMID: 23282474]
[http://dx.doi.org/10.1016/j.aller.2017.09.015] [PMID: 29342408]
[http://dx.doi.org/10.1016/j.ccell.2020.03.012] [PMID: 32289272]
[http://dx.doi.org/10.4172/1948-5956-C1-096]
[http://dx.doi.org/10.1016/j.wneu.2020.03.128] [PMID: 32251831]
[http://dx.doi.org/10.15326/jcopdf.3.1.2015.0160] [PMID: 28848860]
[PMID: 23378757]
[http://dx.doi.org/10.1038/nature03552] [PMID: 15944707]
[http://dx.doi.org/10.1016/j.cell.2008.02.019] [PMID: 18329372]
[http://dx.doi.org/10.1016/j.ydbio.2007.08.007] [PMID: 17765889]
[http://dx.doi.org/10.1159/000500419] [PMID: 31266011]
[http://dx.doi.org/10.1038/ni.3026] [PMID: 25362490]
[http://dx.doi.org/10.1038/leu.2011.305] [PMID: 22116552]
[http://dx.doi.org/10.1158/0008-5472.CAN-10-2412] [PMID: 20940405]
[http://dx.doi.org/10.1183/09031936.00091513] [PMID: 24743967]
[http://dx.doi.org/10.1016/j.joca.2010.05.025] [PMID: 20864019]
[http://dx.doi.org/10.1002/jor.22157] [PMID: 22674437]
[http://dx.doi.org/10.1038/cddis.2014.462] [PMID: 25356868]
[http://dx.doi.org/10.1016/j.brainresbull.2018.02.009] [PMID: 29438779]
[http://dx.doi.org/10.1136/thx.2003.006502] [PMID: 14985567]
[http://dx.doi.org/10.1093/nar/gks1466] [PMID: 23325846]
[http://dx.doi.org/10.1016/j.gpb.2018.06.001] [PMID: 29981854]
[http://dx.doi.org/10.1038/leu.2012.90] [PMID: 22456625]
[http://dx.doi.org/10.2174/1389200220666191021100001] [PMID: 31631818]
[http://dx.doi.org/10.1007/s00005-019-00547-4] [PMID: 31139837]
[http://dx.doi.org/10.1016/j.jaci.2016.01.029] [PMID: 27025347]
[http://dx.doi.org/10.3390/cells8050420] [PMID: 31071965]
[http://dx.doi.org/10.3389/fimmu.2017.00587] [PMID: 28588581]
[http://dx.doi.org/10.1164/rccm.201002-0304OC] [PMID: 21037022]
[http://dx.doi.org/10.1371/journal.pgen.1002242] [PMID: 21935352]
[http://dx.doi.org/10.1189/jlb.1111571] [PMID: 22389313]