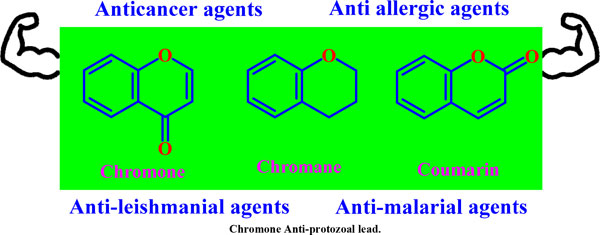
Abstract
Tropical, vector-borne, and neglected diseases with a limited number of medication therapies include Leishmaniasis, Malaria, Chagas and Human African Trypanosomiasis (HAT). Chromones are a large class of heterocyclic compounds with significant applications. This heterocycle has long aroused the interest of scientists and the general public from biosynthetic and synthetic points of view owing to its interesting pharmacological activities. Chromones and their hybrids and isomeric forms proved to be an exciting scaffold to investigate these diseases. The in vitro activities of Chromone, Chromane, and a panel of other related benzopyran class compounds against Trypanosoma brucei rhodesiense, Trypanosoma brucei gambiense, Trypanosoma cruzi, and numerous Leishmanial and Malarial species were investigated in our previous studies. The current article briefly describes the neglected diseases and the current treatment. This review aims to attempt to find better alternatives by scrutinizing natural and synthetic derivatives for which chromones and their analogues were discovered to be a new and highly effective scaffold for the treatment of neglected diseases, including compounds with dual activity or activity against multiple parasites. Additionally, the efficacy of other new scaffolds was also thoroughly examined. This article also discusses prospects for identifying more unique targets for the disease, focusing on flavonoids as drug molecules that are less cytotoxic and high antiprotozoal potential. It also emphasizes the changes that can be made while searching for potential therapies-comparing existing treatments against protozoal diseases and the advantages of the newer chromone analogues over them. Finally, the structure- activity relationship at each atom of the chromone has also been highlighted.
Keywords: Chromone, Chromane, Leishmaniasis, Malaria, Chagas, Human African trypanosomiasis.
[http://dx.doi.org/10.1007/s11908-005-0021-1] [PMID: 15610669]
[http://dx.doi.org/10.1016/j.drudis.2022.04.004] [PMID: 35398562]
[http://dx.doi.org/10.1007/978-3-319-72386-0_1]
[http://dx.doi.org/10.1007/s00436-008-0943-2] [PMID: 18389282]
[http://dx.doi.org/10.1007/s00436-021-07139-2] [PMID: 33825036]
[http://dx.doi.org/10.4269/ajtmh.15-0408] [PMID: 26787156]
[http://dx.doi.org/10.1016/S0035-9203(01)90223-8] [PMID: 11490989]
[http://dx.doi.org/10.3855/jidc.4310] [PMID: 25116660]
[http://dx.doi.org/10.1016/j.drudis.2017.06.004] [PMID: 28647378]
[http://dx.doi.org/10.1177/2049936116646063] [PMID: 27536354]
[http://dx.doi.org/10.1016/j.idc.2012.03.005] [PMID: 22632641]
[http://dx.doi.org/10.1111/j.1365-4632.2010.04558.x] [PMID: 21128917]
[http://dx.doi.org/10.1186/s13071-016-1721-0] [PMID: 27553063]
[http://dx.doi.org/10.1111/j.1529-8019.2009.01272.x] [PMID: 19889134]
[http://dx.doi.org/10.1016/j.idc.2012.03.001] [PMID: 22632640]
[http://dx.doi.org/10.1016/j.apjtm.2016.06.021] [PMID: 27794384]
[http://dx.doi.org/10.1016/j.vetpar.2016.07.011] [PMID: 27523945]
[http://dx.doi.org/10.1136/bmjgh-2018-000709] [PMID: 29736277]
[http://dx.doi.org/10.1586/14787210.4.2.177] [PMID: 16597200]
[http://dx.doi.org/10.1517/14656566.2013.755515] [PMID: 23256501]
[http://dx.doi.org/10.1016/S1995-7645(12)60084-4] [PMID: 22575984]
[http://dx.doi.org/10.1016/j.drup.2004.07.002] [PMID: 15533763]
[http://dx.doi.org/10.1007/s10156-004-0348-9] [PMID: 15614453]
[http://dx.doi.org/10.1007/s00253-020-10856-w] [PMID: 32875362]
[http://dx.doi.org/10.1086/507530] [PMID: 16941377]
[http://dx.doi.org/10.1111/j.1469-0691.2011.03635.x] [PMID: 21933305]
[http://dx.doi.org/10.1517/13543784.17.5.787] [PMID: 18447603]
[http://dx.doi.org/10.1093/qjmed/hct116] [PMID: 23744570]
[http://dx.doi.org/10.1111/j.1742-4658.2007.05997.x] [PMID: 17824953]
[http://dx.doi.org/10.1016/j.cell.2016.07.055] [PMID: 27768886]
[http://dx.doi.org/10.1007/s12098-017-2332-2] [PMID: 28357581]
[http://dx.doi.org/10.1080/00034983.1969.11686625] [PMID: 4190223]
[http://dx.doi.org/10.1038/415680a] [PMID: 11832956]
[http://dx.doi.org/10.1073/pnas.0903423106] [PMID: 19666598]
[http://dx.doi.org/10.1186/cc2183] [PMID: 12930555]
[http://dx.doi.org/10.1146/annurev.med.50.1.179] [PMID: 10073271]
[http://dx.doi.org/10.1136/bmj.1.4293.476] [PMID: 20784787]
[http://dx.doi.org/10.1002/ardp.200700184] [PMID: 18297679]
[http://dx.doi.org/10.1136/bmj.1.6005.323] [PMID: 764937]
[http://dx.doi.org/10.1186/1475-2875-10-144] [PMID: 21609473]
[http://dx.doi.org/10.1517/14740338.1.1.19] [PMID: 12904156]
[http://dx.doi.org/10.5897/AJPPX12.015]
[http://dx.doi.org/10.1016/S1473-3099(07)70187-1] [PMID: 17646028]
[http://dx.doi.org/10.1586/14787210.2.2.181] [PMID: 15482185]
[http://dx.doi.org/10.1016/S0163-7258(00)00115-7] [PMID: 11316521]
[http://dx.doi.org/10.1586/14787210.3.6.849] [PMID: 16307498]
[http://dx.doi.org/10.1038/nature09221] [PMID: 20571554]
[http://dx.doi.org/10.1016/j.idc.2018.10.015] [PMID: 30712757]
[http://dx.doi.org/10.1128/CMR.00005-11] [PMID: 21976603]
[http://dx.doi.org/10.1016/S1473-3099(15)00243-1] [PMID: 26231478]
[http://dx.doi.org/10.1136/pgmj.2006.047357] [PMID: 17148699]
[http://dx.doi.org/10.1186/1475-9292-2-11]
[http://dx.doi.org/10.1016/S0020-7519(01)00153-9] [PMID: 11334932]
[http://dx.doi.org/10.1371/journal.pntd.0006814] [PMID: 30383777]
[http://dx.doi.org/10.1093/jac/dkp357] [PMID: 19819909]
[http://dx.doi.org/10.1080/17512433.2018.1509704] [PMID: 30111183]
[http://dx.doi.org/10.1080/21678707.2021.1933431]
[http://dx.doi.org/10.1016/j.actatropica.2015.12.017] [PMID: 26747009]
[http://dx.doi.org/10.1007/s00415-019-09425-7] [PMID: 31209574]
[http://dx.doi.org/10.1016/j.idc.2012.03.003] [PMID: 22632638]
[http://dx.doi.org/10.1136/bmj.325.7362.475] [PMID: 12202330]
[PMID: 23260189]
[http://dx.doi.org/10.17352/2455-5363.000033]
[http://dx.doi.org/10.1097/QCO.0b013e32833f9fd0] [PMID: 20844428]
[http://dx.doi.org/10.1007/s00436-002-0799-9] [PMID: 12743807]
[http://dx.doi.org/10.1007/s00436-002-0766-5] [PMID: 12811548]
[http://dx.doi.org/10.1016/S0006-2952(00)00477-9] [PMID: 11137702]
[http://dx.doi.org/10.1016/0924-8579(94)90012-4] [PMID: 18611614]
[http://dx.doi.org/10.1016/S0169-4758(99)01560-4] [PMID: 10637579]
[http://dx.doi.org/10.1038/sj.bjp.0707354] [PMID: 17618313]
[http://dx.doi.org/10.1186/1756-3305-3-15] [PMID: 20219092]
[http://dx.doi.org/10.1080/17460441.2020.1801630] [PMID: 32783762]
[http://dx.doi.org/10.1021/jm301172v] [PMID: 22989363]
[http://dx.doi.org/10.1371/journal.pone.0107994] [PMID: 25254495]
[http://dx.doi.org/10.1159/000330856] [PMID: 22024637]
[http://dx.doi.org/10.1007/s00044-017-1846-5]
[http://dx.doi.org/10.1016/B978-0-444-64181-6.00002-4]
[http://dx.doi.org/10.4161/epi.6.7.16315] [PMID: 21610327]
[http://dx.doi.org/10.1016/j.canlet.2004.06.010] [PMID: 15488634]
[http://dx.doi.org/10.1080/10286020.2018.1492565] [PMID: 29973097]
[http://dx.doi.org/10.1016/j.lfs.2019.116797] [PMID: 31472146]
[http://dx.doi.org/10.1016/j.jconrel.2021.03.042] [PMID: 33798663]
[http://dx.doi.org/10.1016/j.cclet.2014.05.021]
[http://dx.doi.org/10.1016/j.fitote.2015.09.018] [PMID: 26393898]
[http://dx.doi.org/10.1016/j.cbi.2010.05.002] [PMID: 20452337]
[http://dx.doi.org/10.1016/j.ejmech.2014.08.011] [PMID: 25147152]
[http://dx.doi.org/10.1016/S0306-3623(96)00421-1] [PMID: 9251891]
[http://dx.doi.org/10.1016/j.biopha.2018.07.079] [PMID: 30119198]
[http://dx.doi.org/10.1016/S0954-6111(89)80195-7] [PMID: 2558400]
[http://dx.doi.org/10.7324/JAPS.2013.31208]
[http://dx.doi.org/10.3389/fphar.2020.00073]
[http://dx.doi.org/10.1136/bmj.1.5643.552] [PMID: 4885026]
[http://dx.doi.org/10.1007/s10151-015-1302-9] [PMID: 25893991]
[http://dx.doi.org/10.1159/000249760] [PMID: 6628802]
[http://dx.doi.org/10.1111/j.1745-7262.2006.00197.x] [PMID: 16751992]
[PMID: 25807422]
[http://dx.doi.org/10.3390/molecules190913251] [PMID: 25170948]
[http://dx.doi.org/10.1007/s11033-020-05887-5] [PMID: 33021720]
[http://dx.doi.org/10.1007/s00044-016-1729-1]
[http://dx.doi.org/10.1016/j.bmcl.2015.12.085] [PMID: 26778149]
[http://dx.doi.org/10.3390/molecules22122041] [PMID: 29186046]
[http://dx.doi.org/10.3390/molecules23010070] [PMID: 29286346]
[http://dx.doi.org/10.1021/tx200233c] [PMID: 21809846]
[http://dx.doi.org/10.1155/2014/903529] [PMID: 24949478]
[http://dx.doi.org/10.3390/molecules25040800] [PMID: 32059518]
[http://dx.doi.org/10.1016/j.bioorg.2014.10.006] [PMID: 25462990]
[http://dx.doi.org/10.2306/scienceasia1513-1874.2019.45.221]
[http://dx.doi.org/10.1016/j.bmc.2022.116629] [PMID: 35091169]
[http://dx.doi.org/10.1016/j.tet.2019.130646]
[http://dx.doi.org/10.1002/ptr.5362] [PMID: 25943035]
[http://dx.doi.org/10.21577/1984-6835.20200136]
[http://dx.doi.org/10.3390/molecules25020397] [PMID: 31963596]
[http://dx.doi.org/10.4155/fmc.13.147] [PMID: 24175743]
[http://dx.doi.org/10.3390/molecules26237067] [PMID: 34885649]
[http://dx.doi.org/10.24820/ark.5550190.p011.356]
[http://dx.doi.org/10.1021/acs.jmedchem.6b00698] [PMID: 27411733]
[http://dx.doi.org/10.1016/j.jep.2011.06.015] [PMID: 21708240]
[http://dx.doi.org/10.1016/j.exppara.2010.10.011] [PMID: 20971104]
[http://dx.doi.org/10.1186/s12906-015-0562-2] [PMID: 25886869]
[http://dx.doi.org/10.3390/molecules22030426] [PMID: 28282886]
[http://dx.doi.org/10.1055/s-0032-1328298] [PMID: 23512498]
[http://dx.doi.org/10.1039/C5MD00481K]
[http://dx.doi.org/10.1128/AAC.50.4.1352-1364.2006] [PMID: 16569852]
[http://dx.doi.org/10.1002/ptr.3404] [PMID: 21796699]