Abstract
Background: Hepatic fibrosis is the major issue in chronic liver diseases such as chronic hepatitis C virus (HCV). The newly approved direct acting antiviral (DAA) agents such as Sofosbuvir (SOF) and daclatasvir (DAC) have been found to be associated with decreased fibrotic markers in HCV patients.
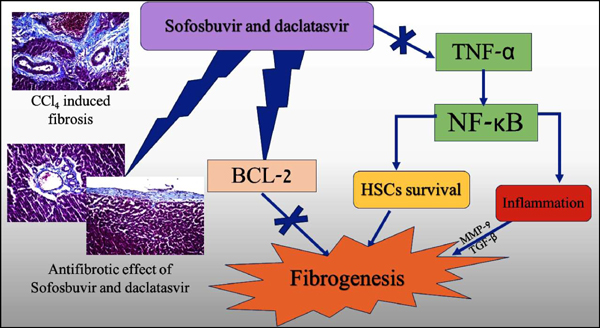
Aim: This study tried to explore whether the reported antifibrotic effect of these drugs is antiviral dependent or drug induced.
Method: Hepatic fibrosis was induced by (0.5ml/kg) CCl4 IP twice a week for six weeks. SOF (20 mg/kg/d) and DAC (30 mg/kg/d) were added in the last four weeks of treatments. Liver functions, fibrotic markers such as Hyaluronic acid and metalloproteinase-9 were detected using immunoassay. The expression of TNF-α/NF-κB signaling pathway as well as Bcl-2 were done using immunoassay.
Results: SOF and DAC exerted a potent antifibrotic effect evidenced by their activity against hyaluronic acid HA and metalloproteinase MMP-9 significantly (P≤0.001). This effect was further proved histopathologically where liver tissues from rats treated by drugs showed marked inhibition of collagen precipitation as well as inhibition of HSCs activation. This antifibrotic action was associated with decreased expression of TNF-α /NF-κB signaling pathway and induction of Bcl-2.
Conclusion: SOF/ DAC antifibrotic effect is independent of its antiviral activity. The molecular events associated with this effect were the downregulation of TNF-α / NF-κB signaling pathway and induction of Bcl-2.
Keywords: Sofosbuvir, daclatasvir, TNF-α, NF-κB, hepatic fibrosis, Bcl-2.
[http://dx.doi.org/10.1177/0091270003258669 ] [PMID: 14681338]
[http://dx.doi.org/10.3892/ijmm.2017.2997 ] [PMID: 28534957]
[http://dx.doi.org/10.1053/j.gastro.2003.11.018 ] [PMID: 14762790]
[http://dx.doi.org/10.1073/pnas.171311298 ] [PMID: 11481452]
[http://dx.doi.org/10.1016/S0098-2997(00)00004-2 ] [PMID: 10978499]
[http://dx.doi.org/10.3748/wjg.v20.i32.11033 ] [PMID: 25170193]
[http://dx.doi.org/10.1016/S0140-6736(13)62121-2 ] [PMID: 24209977]
[http://dx.doi.org/10.1056/NEJMoa1306218 ] [PMID: 24428467]
[http://dx.doi.org/10.2147/IDR.S160593]
[http://dx.doi.org/10.1080/17441692.2014.984742 ] [PMID: 25469976]
[PMID: 28039923]
[http://dx.doi.org/10.1016/j.rmu.2017.05.005]
[http://dx.doi.org/10.5009/gnl17298 ] [PMID: 29409309]
[http://dx.doi.org/10.1111/jgh.13758 ] [PMID: 28177543]
[http://dx.doi.org/10.5604/01.3001.0010.8662 ] [PMID: 29469035]
[http://dx.doi.org/10.1038/s41598-017-09797-8 ] [PMID: 28842610]
[http://dx.doi.org/10.1007/s00535-015-1108-6 ] [PMID: 26208695]
[http://dx.doi.org/10.1093/ajcp/28.1.56 ] [PMID: 13458125]
[http://dx.doi.org/10.1016/0168-8278(95)80226-6 ] [PMID: 7560864]
[http://dx.doi.org/10.1016/S0168-8278(02)00429-4 ] [PMID: 12591185]
[http://dx.doi.org/10.5114/ceh.2018.78121 ] [PMID: 30324142]
[http://dx.doi.org/10.1016/j.jhep.2015.07.041 ] [PMID: 26325535]
[http://dx.doi.org/10.1016/S0168-8278(18)31042-0]
[http://dx.doi.org/10.1159/000084626 ] [PMID: 15785038]
[http://dx.doi.org/10.1074/jbc.M700554200 ] [PMID: 17322299]
[http://dx.doi.org/10.1016/j.cyto.2004.11.001 ] [PMID: 15760680]
[http://dx.doi.org/10.1016/S0168-8278(99)80007-5 ] [PMID: 9927150]
[http://dx.doi.org/10.1002/hep.26429 ] [PMID: 23553591]
[http://dx.doi.org/10.1038/nature04870 ] [PMID: 16724054]
[http://dx.doi.org/10.1016/j.jhep.2016.06.019 ] [PMID: 27388925]
[http://dx.doi.org/10.1016/j.jhep.2016.07.004 ] [PMID: 27417216]
[http://dx.doi.org/10.1016/j.jhep.2016.04.008 ] [PMID: 27084592]
[PMID: 28035203]
[http://dx.doi.org/10.1002/hep.29855 ] [PMID: 29476694]
[http://dx.doi.org/10.1053/j.gastro.2019.01.027 ] [PMID: 30660729]