Abstract
Background: Anti-tumor necrosis factor-α (TNF-α) is a life-changing treatment leading to quality-of-life improvement. Nonetheless, this treatment is associated with a high risk of infection, especially tuberculosis.
Objective: Our study aimed to determine the frequency of active tuberculosis in our patients with chronic rheumatic disease and treated with TNF-α.
Methods: We conducted a retrospective study including patients with Rheumatoid Arthritis and Spondylarthritis diagnosed according to ACR/EULAR 2009 criteria and ASAS 2010, respectively, and treated with biological agents for at least 6 months. We collected data regarding tuberculosis screening and the occurrence of active tuberculosis during follow-up.
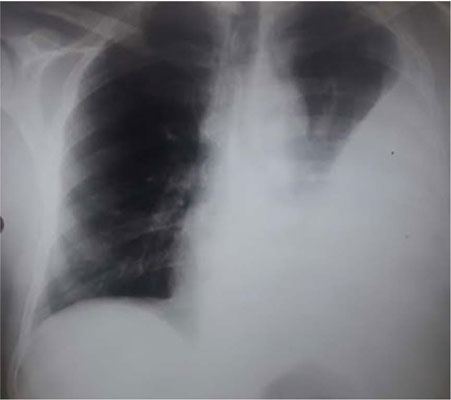
Results: 82 patients were included (37 men and 45 women). The mean age was 42 ± 3.4 years. At inclusion, no patient had a medical history of tuberculosis. The diagnosis of latent tuberculosis infection was established in 17 patients (20.7%). Prophylactic treatment was prescribed in all these cases for three months. Two cases (2.4%) of active tuberculosis occurred under biologic (infliximab). It was two severe forms of tuberculosis. The first case had miliary tuberculosis associated with hepatic and peritoneal involvement. The second one had pleural tuberculosis. These two patients received anti-tuberculosis therapy, and the biological treatment was interrupted. Given the high disease activity, the anti-TNF-α was restarted after 3 and 4 months. There was no recurrence of tuberculosis after 7 years of follow-up.
Conclusion: The use of TNF-α blockers is associated with a risk of disseminated forms of tuberculosis. Tuberculosis screening, which is recommended before the biological onset, is also necessary under this treatment. Restarting the anti-TNF-α after appropriate treatment of tuberculosis seemed to be safe.
Keywords: Anti-tumor necrosis factor-α, tuberculosis, chronic rheumatic disease, Rheumatoid Arthritis, Spondylarthritis, prophylactic treatment.
[http://dx.doi.org/10.2147/DHPS.S28801] [PMID: 23569399]
[http://dx.doi.org/10.1016/j.jmii.2013.03.005] [PMID: 23727394]
[http://dx.doi.org/10.1016/S1473-3099(03)00545-0] [PMID: 12614731]
[http://dx.doi.org/10.1002/art.27584] [PMID: 20872595]
[http://dx.doi.org/10.1136/ard.2009.108217] [PMID: 19297345]
[http://dx.doi.org/10.1136/ard.2009.108233] [PMID: 19297344]
[http://dx.doi.org/10.1016/j.therap.2017.02.002] [PMID: 28318613]
[http://dx.doi.org/10.1056/NEJMoa011110] [PMID: 11596589]
[http://dx.doi.org/10.1183/09031936.00028510] [PMID: 20530046]
[http://dx.doi.org/10.1056/NEJMcp1005750] [PMID: 21488766]
[http://dx.doi.org/10.1093/rheumatology/kei103] [PMID: 16172152]
[http://dx.doi.org/10.1086/424455] [PMID: 15486857]
[http://dx.doi.org/10.1038/ncprheum0336] [PMID: 17075599]
[http://dx.doi.org/10.1136/ard.2009.118935] [PMID: 19854715]
[http://dx.doi.org/10.1016/j.jinf.2014.10.019] [PMID: 25452040]
[http://dx.doi.org/10.1186/1465-9921-7-56] [PMID: 16579856]
[http://dx.doi.org/10.1097/BOR.0b013e3283474d62] [PMID: 21519268]
[http://dx.doi.org/10.1080/14787210.2018.1483238] [PMID: 29848120]
[http://dx.doi.org/10.1111/1756-185X.13129] [PMID: 28730751]
[http://dx.doi.org/10.1002/acr.21641] [PMID: 22473917]