Abstract
Background: Osteosarcoma is the most common type of primary malignant bone tumor.
Introduction: This study aimed to explore potential key prognostic genes and their roles in osteosarcoma.
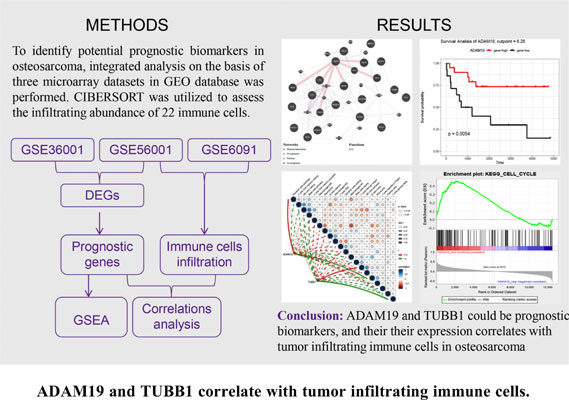
Methods: Three microarray datasets for osteosarcoma were downloaded from the GEO database. Differentially expressed genes (DEGs) were screened by the Limma package. Functional enrichment analysis was performed based on DAVID, GeneMANIA, and Metascape databases. Prognostic value of DEGs was elevated by survival analysis. CIBERSORT was used to assess the infiltrating abundance of 22 immune cells, followed by the Pearson correlation analysis between immune cells and prognosis-related genes. Gene set enrichment analysis and drug-gene interactions prediction were performed for prognosis-related genes.
Results: A total of 8 common up-regulated DEGs and 13 common down-regulated DEGs were screened in the GSE36001 and GSE56001 datasets. Enrichment analysis showed these DEGs were implicated in platelet activation, SMAD protein phosphorylation, lymphocyte/leukocyte/T cells activation, and cell migration. Survival analysis indicated that elevated expression of ADAM19 and TUBB1 were associated with a favorable prognosis. CIBERSORT algorithm revealed the higher infiltrating level of CD8 T cells, macrophages M0, and M2 in osteosarcoma. ADAM19 expression positively correlated with naïve B cells and negatively correlated with activated dendritic cells infiltrating abundance. TUBB1 expression positively correlated with gamma delta T cells while negatively correlated with helper follicular T cells infiltrating abundance. A total of 56 drugs were found to target TUBB1.
Conclusion: ADAM19 and TUBB1 could be prognostic biomarkers in osteosarcoma. Both their expression correlates with tumor infiltrating immune cells. TUBB1 was a multi-drug target that might be a therapeutic target in osteosarcoma.
Keywords: Osteosarcoma, predicts prognosis, ADAM19, TUBB1, prognostic biomarkers, leukocyte.
[http://dx.doi.org/10.1080/07357907.2020.1768401] [PMID: 32400205]
[http://dx.doi.org/10.1007/s40744-016-0050-2] [PMID: 27933467]
[http://dx.doi.org/10.1007/s00264-019-04348-4] [PMID: 31127366]
[http://dx.doi.org/10.1080/14737140.2018.1413939] [PMID: 29210294]
[http://dx.doi.org/10.1016/j.ocl.2015.08.022] [PMID: 26614941]
[http://dx.doi.org/10.1186/s13018-019-1301-z] [PMID: 31484533]
[http://dx.doi.org/10.3892/mmr.2019.10104] [PMID: 30942427]
[http://dx.doi.org/10.1016/j.cellimm.2017.10.011] [PMID: 29117898]
[PMID: 30556159]
[http://dx.doi.org/10.1186/s12885-020-07536-3] [PMID: 33087099]
[http://dx.doi.org/10.1186/gb-2003-4-5-p3] [PMID: 12734009]
[http://dx.doi.org/10.1186/gb-2008-9-s1-s4]
[http://dx.doi.org/10.1038/s41467-019-09234-6] [PMID: 30944313]
[http://dx.doi.org/10.1038/nmeth.3337] [PMID: 25822800]
[http://dx.doi.org/10.1093/nar/gkx1143] [PMID: 29156001]
[http://dx.doi.org/10.1093/jb/mvs108] [PMID: 22992842]
[http://dx.doi.org/10.1111/cas.12464] [PMID: 24974736]
[http://dx.doi.org/10.1139/cjpp-2016-0522] [PMID: 28177668]
[http://dx.doi.org/10.1016/j.cbi.2019.05.005] [PMID: 31059706]
[http://dx.doi.org/10.1002/cphy.c110012] [PMID: 23720251]
[http://dx.doi.org/10.1007/s00018-016-2210-5] [PMID: 27022944]
[http://dx.doi.org/10.1016/j.mam.2008.08.001] [PMID: 18762209]
[http://dx.doi.org/10.4155/fmc-2021-0177]
[http://dx.doi.org/10.1016/j.canlet.2019.10.003] [PMID: 31593799]
[http://dx.doi.org/10.1002/ijc.31405] [PMID: 29582409]
[http://dx.doi.org/10.1186/s12885-016-2178-4] [PMID: 26912236]
[http://dx.doi.org/10.1080/09537104.2017.1411587] [PMID: 29333906]
[http://dx.doi.org/10.1182/bloodadvances.2020004057]
[http://dx.doi.org/10.15252/emmm.201809569] [PMID: 30446499]
[http://dx.doi.org/10.1016/j.canlet.2018.10.011] [PMID: 30343113]
[http://dx.doi.org/10.3390/ijms21155207] [PMID: 32717819]
[http://dx.doi.org/10.1016/j.cancergen.2021.12.010] [PMID: 35007853]
[http://dx.doi.org/10.1038/517139a] [PMID: 25567266]
[http://dx.doi.org/10.1080/2162402X.2019.1571388] [PMID: 30906667]
[http://dx.doi.org/10.1007/s11999-010-1302-z] [PMID: 20232181]
[http://dx.doi.org/10.3892/ol.2016.4175] [PMID: 26998143]
[http://dx.doi.org/10.1089/dna.2017.3669] [PMID: 28650673]