Abstract
Cancer is a leading cause of death and a severe threat to global public health. Organoid, as a novel 3D in vitro model, has been applied in various tumor related studies due to its apparent advantages. The organoid is mainly constructed by Matrigel-depended 3D culture system, Air-Liquid Interface (ALI) culture, and Microfluidic culture or Organ-on-chips platform. For the application in carcinogenesis studies, the organoid model may favor depicting initiative hallmarks and identifying potential intervening targets, investigating driver genes of carcinogenesis, and identifying known or unknown risk or protective factors.
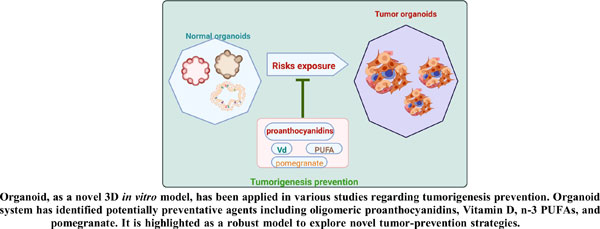
In this review, we discussed different organoid construction methods and their properties. We also noted that tumor organoids can portray initiative hallmarks and identify possible intervening targets, as well as explore carcinogenesis driver genes and uncover known or unknown risks or protective factors. Organoid systems have been used to identify tumor-preventive drugs such as oligomeric proanthocyanidins, Vitamin D, n-3 PUFAs, and pomegranate. The current evidence underscores the organoid model's potential importance in developing innovative tumorprevention techniques.
Keywords: Organoid, 3D model, tumorigenesis, pharmacology, oncogenic prevention, cancer.
[http://dx.doi.org/10.3322/caac.21708] [PMID: 35020204]
[http://dx.doi.org/10.1016/j.tibtech.2017.12.005] [PMID: 29366522]
[http://dx.doi.org/10.1186/s13046-022-02280-x] [PMID: 35139880]
[http://dx.doi.org/10.1016/j.gde.2015.02.007] [PMID: 25796043]
[http://dx.doi.org/10.1038/s41416-020-0993-5] [PMID: 32728094]
[http://dx.doi.org/10.1016/j.wjorl.2016.05.007] [PMID: 28642939]
[http://dx.doi.org/10.3389/fmed.2018.00085] [PMID: 29682505]
[http://dx.doi.org/10.1038/nrurol.2017.65] [PMID: 28534535]
[http://dx.doi.org/10.1016/j.celrep.2013.08.022] [PMID: 24055055]
[http://dx.doi.org/10.1016/j.bbcan.2020.188350] [PMID: 32007597]
[http://dx.doi.org/10.1158/1055-9965.EPI-15-0578] [PMID: 26667886]
[http://dx.doi.org/10.1016/j.biotechadv.2017.10.005] [PMID: 29056474]
[http://dx.doi.org/10.1038/nm.3585] [PMID: 24859528]
[http://dx.doi.org/10.1038/nm.3802] [PMID: 25706875]
[http://dx.doi.org/10.1038/nature14415] [PMID: 25924068]
[http://dx.doi.org/10.1038/nm.4355] [PMID: 28628110]
[http://dx.doi.org/10.1126/science.aao3130] [PMID: 28912133]
[http://dx.doi.org/10.1053/j.gastro.2011.07.050] [PMID: 21889923]
[http://dx.doi.org/10.1245/s10434-018-6662-8] [PMID: 30003451]
[http://dx.doi.org/10.1016/j.celrep.2018.07.001] [PMID: 30067989]
[http://dx.doi.org/10.1016/j.gie.2017.12.032] [PMID: 29325707]
[http://dx.doi.org/10.1038/nm.4438] [PMID: 29131160]
[http://dx.doi.org/10.1073/pnas.1701219114] [PMID: 28270604]
[http://dx.doi.org/10.1016/j.celrep.2019.02.063] [PMID: 30893594]
[http://dx.doi.org/10.1038/ng.2983] [PMID: 24816253]
[http://dx.doi.org/10.1016/j.cell.2014.12.021] [PMID: 25557080]
[http://dx.doi.org/10.1586/14737140.2015.1003046] [PMID: 25603995]
[http://dx.doi.org/10.1016/j.cell.2017.11.010] [PMID: 29224780]
[http://dx.doi.org/10.1038/s41467-018-05190-9] [PMID: 30061675]
[http://dx.doi.org/10.1038/s41587-019-0048-8] [PMID: 30833775]
[http://dx.doi.org/10.15252/embj.2018100300] [PMID: 30643021]
[http://dx.doi.org/10.1038/nm.3175] [PMID: 23584089]
[http://dx.doi.org/10.1038/nm.3967] [PMID: 26457759]
[http://dx.doi.org/10.1152/ajpcell.00117.2015] [PMID: 26289750]
[http://dx.doi.org/10.1016/j.stem.2016.04.003] [PMID: 27212702]
[http://dx.doi.org/10.1158/1078-0432.CCR-10-1204] [PMID: 21159886]
[http://dx.doi.org/10.1146/annurev-pathol-012615-044249] [PMID: 26907527]
[http://dx.doi.org/10.1016/j.cell.2018.03.017] [PMID: 29625057]
[http://dx.doi.org/10.1016/j.celrep.2016.12.016] [PMID: 28052255]
[http://dx.doi.org/10.1186/s12885-018-4238-4] [PMID: 29587663]
[http://dx.doi.org/10.1038/nrc3368] [PMID: 23051844]
[http://dx.doi.org/10.1016/j.neo.2014.12.004] [PMID: 25622895]
[http://dx.doi.org/10.21037/atm.2019.12.88] [PMID: 32175407]
[http://dx.doi.org/10.1016/j.it.2020.06.010] [PMID: 32654925]
[http://dx.doi.org/10.3389/fimmu.2020.603640] [PMID: 33362787]
[http://dx.doi.org/10.1016/j.stem.2018.11.016] [PMID: 30526881]
[http://dx.doi.org/10.1038/nature07935] [PMID: 19329995]
[http://dx.doi.org/10.1016/j.cell.2018.07.009] [PMID: 30100188]
[http://dx.doi.org/10.1038/nm.1951] [PMID: 19398967]
[http://dx.doi.org/10.1126/science.aao2774] [PMID: 29472484]
[http://dx.doi.org/10.1016/j.cell.2018.11.021] [PMID: 30550791]
[http://dx.doi.org/10.1038/nature05058] [PMID: 16871203]
[http://dx.doi.org/10.1002/bit.24944] [PMID: 23616255]
[http://dx.doi.org/10.1021/acs.langmuir.9b01163] [PMID: 31291112]
[http://dx.doi.org/10.2217/fmb-2019-0345] [PMID: 33215543]
[http://dx.doi.org/10.1136/gutjnl-2017-315920] [PMID: 29666172]
[http://dx.doi.org/10.1053/j.gastro.2014.09.042] [PMID: 25307862]
[http://dx.doi.org/10.1016/j.cell.2018.07.027] [PMID: 30096312]
[http://dx.doi.org/10.1038/nature13480] [PMID: 25079317]
[http://dx.doi.org/10.1053/j.gastro.2020.04.033] [PMID: 32325086]
[http://dx.doi.org/10.1038/s41588-019-0574-9] [PMID: 32025000]
[http://dx.doi.org/10.1038/ncomms14686] [PMID: 28272465]
[http://dx.doi.org/10.1016/j.stem.2017.12.009] [PMID: 29337182]
[http://dx.doi.org/10.1053/j.seminoncol.2015.09.007] [PMID: 26970122]
[http://dx.doi.org/10.1016/j.molmed.2017.02.007] [PMID: 28341301]
[http://dx.doi.org/10.1128/IAI.02561-14] [PMID: 25312952]
[http://dx.doi.org/10.3390/ijms20204981] [PMID: 31600949]
[http://dx.doi.org/10.1152/ajpgi.00052.2015] [PMID: 25977509]
[http://dx.doi.org/10.1053/j.gastro.2018.06.049] [PMID: 29964038]
[http://dx.doi.org/10.1093/carcin/bgy034] [PMID: 29684110]
[http://dx.doi.org/10.1038/s41598-018-21478-8] [PMID: 29463813]
[http://dx.doi.org/10.1089/ars.2019.7911] [PMID: 32940048]
[http://dx.doi.org/10.1093/carcin/bgt412] [PMID: 24336194]
[http://dx.doi.org/10.1111/j.1600-0625.2008.00829.x] [PMID: 19320737]
[http://dx.doi.org/10.1080/19490976.2020.1734423] [PMID: 32138622]
[http://dx.doi.org/10.1186/s12935-020-01723-9] [PMID: 33407519]
[http://dx.doi.org/10.3390/cancers11091359] [PMID: 31540244]
[http://dx.doi.org/10.1136/gutjnl-2020-320946] [PMID: 32641470]
[http://dx.doi.org/10.1097/TP.0000000000002647] [PMID: 30747839]
[http://dx.doi.org/10.1039/C7NR06573F] [PMID: 29303193]
[http://dx.doi.org/10.1017/S0007114509992741] [PMID: 20003617]
[http://dx.doi.org/10.1359/jbmr.07s206] [PMID: 18290728]
[http://dx.doi.org/10.1038/nrc3691] [PMID: 24705652]
[http://dx.doi.org/10.1111/febs.14998] [PMID: 31306552]
[http://dx.doi.org/10.1016/j.jsbmb.2017.02.001] [PMID: 28163244]
[http://dx.doi.org/10.1034/j.1600-0781.2003.00019.x] [PMID: 12945805]
[http://dx.doi.org/10.1053/sonu.2003.50004] [PMID: 12638382]
[http://dx.doi.org/10.1016/j.jep.2006.09.006] [PMID: 17157465]
[http://dx.doi.org/10.1158/0008-5472.CAN-06-3941] [PMID: 17389758]
[http://dx.doi.org/10.1053/j.gastro.2014.12.036] [PMID: 25575570]
[http://dx.doi.org/10.1016/B978-0-12-800100-4.00003-9] [PMID: 24388214]
[http://dx.doi.org/10.1038/nmicrobiol.2017.8] [PMID: 28225000]