Abstract
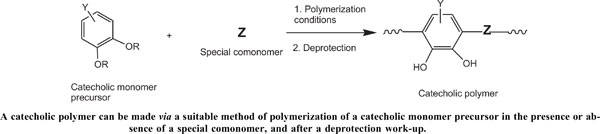
Over the last few years, research on catechol-containing polymers has focused mainly on making mussel-inspired catechol-containing polymers and examining their adhesion ability onto various substrata under dry and wet conditions. Indeed, a surge of dopamine-bearing vinylic monomers such as dopamine acrylates and their protected ones have been homopolymerized or copolymerized with fittingly chosen comonomers for targeted applications. Novel polymerization methods such as RAFT and ATRP have been gratifyingly employed to realize these polymers with controlled molecular weights and polydispersity indexes. The protection of hydroxyl groups of the dopamine-based vinyl derivatives has been achieved with different groups, namely, alkyl, benzyl, acetal, silyl, and ester. Nevertheless, in several cases, the unprotected dopamine-based vinylic monomers have been unprecedentedly shown to undergo polymerization with no inhibition or retardation. Ring-opening polymerization has been applied to copolymerizing several oxiranecontaining dopamine monomers and catechol-containing monomers with cyclic comonomers with no major difficulty. Polymers from this method exhibited excellent scaffolds for preparing various materials with desired functions such as electronic conductivity and adhesion to a wide range of objects. Catechol and catechol-containing molecules have been subjected to polycondensation with a number of comonomers, such as formaldehyde, polyamines, polyols, and polyacids, polyisocyanates, under special conditions. These polycondensation resins have been evaluated mainly for their adsorption capacity towards heavy metals and dyes for wastewater decontamination. Proteins antifouling properties of some of these resins have been demonstrated as well. Their special chemistry allowed their use in realizing metal nanoparticles for different purposes.
Keywords: Adhesion, catechol, mussel-inspired polymer, polyaddition, polycondensation, ring-opening polymerization.
[http://dx.doi.org/10.1016/j.crci.2008.05.011]
[http://dx.doi.org/10.1021/ja01170a009]
[http://dx.doi.org/10.1021/ja01170a010]
[http://dx.doi.org/10.1021/ja00897a025]
[http://dx.doi.org/10.1039/9781788019743]
[http://dx.doi.org/10.1046/j.1462-2920.2001.00176.x] [PMID: 11321547]
[http://dx.doi.org/10.1126/science.212.4498.1038] [PMID: 17779975]
[http://dx.doi.org/10.1080/15583724.2014.881373]
[http://dx.doi.org/10.13005/ojc/340301]
[http://dx.doi.org/10.2174/2452271602666180910141623]
[http://dx.doi.org/10.3390/molecules24142586] [PMID: 31315269]
[http://dx.doi.org/10.1039/C9CS00849G] [PMID: 32393930]
[http://dx.doi.org/10.1039/C9NR09780E] [PMID: 31907498]
[http://dx.doi.org/10.1002/pola.28368] [PMID: 27917020]
[http://dx.doi.org/10.2217/nnm.15.89] [PMID: 26377046]
[http://dx.doi.org/10.1002/adma.201704640] [PMID: 29356146]
[http://dx.doi.org/10.1126/science.aab0556] [PMID: 26250681]
[http://dx.doi.org/10.1021/acsbiomaterials.7b00673] [PMID: 33445356]
[http://dx.doi.org/10.1007/978-981-10-6077-9_16]
[http://dx.doi.org/10.1016/j.progpolymsci.2017.09.002]
[http://dx.doi.org/10.3390/molecules26030559] [PMID: 33494541]
[http://dx.doi.org/10.1016/j.jdent.2020.103404] [PMID: 32522689]
[http://dx.doi.org/10.1016/j.dental.2021.01.005] [PMID: 33579531]
[http://dx.doi.org/10.3390/polym13193317] [PMID: 34641133]
[http://dx.doi.org/10.1002/polc.5070740120]
[http://dx.doi.org/10.1007/s10924-020-01766-z]
[http://dx.doi.org/10.1039/C9PY00846B]
[http://dx.doi.org/10.1021/acsami.8b19489] [PMID: 30499653]
[http://dx.doi.org/10.1039/C9TA12614G]
[http://dx.doi.org/10.1016/j.arabjc.2013.03.012]
[http://dx.doi.org/10.1155/2020/8843162]
[http://dx.doi.org/10.1080/01496395.2015.1012591]
[http://dx.doi.org/10.1016/j.seppur.2017.11.013]
[http://dx.doi.org/10.1016/j.ecoenv.2016.03.037] [PMID: 27057995]
[http://dx.doi.org/10.1021/acssuschemeng.6b02593]
[http://dx.doi.org/10.1016/j.carbpol.2019.01.046] [PMID: 30732811]
[http://dx.doi.org/10.1016/j.powtec.2017.01.013]
[http://dx.doi.org/10.1007/s12221-020-1161-5]
[http://dx.doi.org/10.1016/j.apsusc.2019.145080]
[http://dx.doi.org/10.1002/anie.202015833] [PMID: 33507605]
[http://dx.doi.org/10.1002/admi.201700506]
[http://dx.doi.org/10.3390/polym12112727] [PMID: 33213051]
[http://dx.doi.org/10.1039/C7GC03323K]
[http://dx.doi.org/10.1021/acs.macromol.6b02213]
[http://dx.doi.org/10.1016/j.eurpolymj.2019.01.063]
[http://dx.doi.org/10.1111/jicd.12054] [PMID: 23857900]
[http://dx.doi.org/10.1016/j.polymer.2019.01.012]
[http://dx.doi.org/10.3390/polym13040666] [PMID: 33672307]
[http://dx.doi.org/10.1021/acs.biomac.6b01613] [PMID: 28140560]
[http://dx.doi.org/10.1021/acsami.0c03133] [PMID: 32227990]
[http://dx.doi.org/10.1039/C5PY01234A]
[http://dx.doi.org/10.1002/marc.202000055] [PMID: 32297374]
[http://dx.doi.org/10.1016/j.apsusc.2019.144414]
[http://dx.doi.org/10.1021/acssuschemeng.8b03429]
[http://dx.doi.org/10.1021/acs.macromol.7b00217]
[http://dx.doi.org/10.1039/C9RA04719K] [PMID: 35521353]
[http://dx.doi.org/10.1021/acs.macromol.5b02441]
[http://dx.doi.org/10.1039/C5PY00127G]
[http://dx.doi.org/10.1021/acs.macromol.6b01857]
[http://dx.doi.org/10.1002/adma.201703373] [PMID: 28869678]
[http://dx.doi.org/10.1021/acs.chemmater.8b02307]
[http://dx.doi.org/10.1021/acsaem.9b00443]
[http://dx.doi.org/10.1039/D0SE00531B]
[http://dx.doi.org/10.1016/j.progpolymsci.2018.04.002]
[http://dx.doi.org/10.1021/acsomega.8b02768] [PMID: 31458294]
[http://dx.doi.org/10.1016/j.compositesb.2019.107687]
[http://dx.doi.org/10.1021/acsapm.9b01179]
[http://dx.doi.org/10.1021/acsomega.0c00259] [PMID: 32280892]
[http://dx.doi.org/10.1021/acs.biomac.5b00451] [PMID: 26176305]
[http://dx.doi.org/10.1039/D0PY00810A]
[http://dx.doi.org/10.1039/C5TA09437B]
[http://dx.doi.org/10.1039/C4CC08366K] [PMID: 25500961]
[http://dx.doi.org/10.1002/macp.201500504]
[http://dx.doi.org/10.1002/jbm.a.35633] [PMID: 26714824]
[http://dx.doi.org/10.1021/acsapm.0c00682]
[http://dx.doi.org/10.1021/acs.macromol.9b01405]
[http://dx.doi.org/10.1021/acs.macromol.6b00831]
[http://dx.doi.org/10.1002/macp.201600553]
[http://dx.doi.org/10.1039/D0PY00854K]
[http://dx.doi.org/10.1016/j.compositesa.2021.106477]
[http://dx.doi.org/10.1039/C7RA08769A]
[http://dx.doi.org/10.1002/pi.6175]
[http://dx.doi.org/10.1021/acsaem.0c03244]
[http://dx.doi.org/10.1021/acssuschemeng.8b04400]
[http://dx.doi.org/10.1002/pola.29453]
[http://dx.doi.org/10.1021/acs.macromol.7b00970]
[http://dx.doi.org/10.1016/j.polymer.2019.121667]
[http://dx.doi.org/10.1002/macp.202100378]
[http://dx.doi.org/10.1039/C4PY01790K]
[http://dx.doi.org/10.1007/s42235-018-0037-5]
[http://dx.doi.org/10.1021/acs.macromol.7b01304]
[http://dx.doi.org/10.1021/acsmacrolett.7b00837] [PMID: 35610919]
[http://dx.doi.org/10.1039/D0SM01944E] [PMID: 33438707]
[http://dx.doi.org/10.1021/acsapm.0c00699]
[http://dx.doi.org/10.1039/C5TB00949A] [PMID: 32262550]
[http://dx.doi.org/10.1021/acsami.6b04075] [PMID: 27377859]
[http://dx.doi.org/10.2494/photopolymer.31.75]
[http://dx.doi.org/10.1039/C7RA01778B]
[http://dx.doi.org/10.1021/acs.iecr.5b00171]
[http://dx.doi.org/10.1016/j.cej.2020.126340] [PMID: 32848507]
[http://dx.doi.org/10.1039/C7CC07215E] [PMID: 29052668]
[http://dx.doi.org/10.1039/C9RA10606E] [PMID: 35492658]
[http://dx.doi.org/10.1021/acsapm.9b00443]
[http://dx.doi.org/10.1246/cl.190305]
[http://dx.doi.org/10.1021/ja303369p] [PMID: 22582754]
[http://dx.doi.org/10.1021/acsami.7b00270] [PMID: 28177600]
[http://dx.doi.org/10.1039/C8PY01831F]