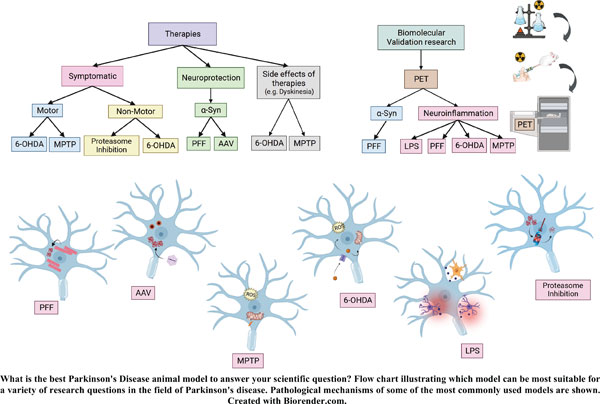
Abstract
Parkinson’s disease (PD) is a debilitating neurodegenerative multisystem disorder leading to motor and non-motor symptoms in millions of individuals. Despite intense research, there is still no cure, and early disease biomarkers are lacking. Animal models of PD have been inspired by basic elements of its pathogenesis, such as dopamine dysfunction, alpha-synuclein accumulation, neuroinflammation and disruption of protein degradation, and these have been crucial for a deeper understanding of the mechanisms of pathology, the identification of biomarkers, and evaluation of novel therapies. Imaging biomarkers are non-invasive tools to assess disease progression and response to therapies; their discovery and validation have been an active field of translational research. Here, we highlight different considerations of animal models of PD that can be applied to future research, in terms of their suitability to answer different research questions. We provide the reader with important considerations of the best choice of model to use based on the disease features of each model, including issues related to different species. In addition, positron emission tomography studies conducted in PD animal models in the last 5 years are presented. With a variety of different species, interventions and genetic information, the choice of the most appropriate model to answer research questions can be daunting, especially since no single model recapitulates all aspects of this complex disorder. Appropriate animal models in conjunction with in vivo molecular imaging tools, if selected properly, can be a powerful combination for the assessment of novel therapies and developing tools for early diagnosis.
Keywords: Animal models, Parkinson’s disease, rodent, non-human primate, minipig, alpha-synuclein, positron emission tomography, autoradiography.
[http://dx.doi.org/10.1038/nrdp.2017.13] [PMID: 28332488]
[http://dx.doi.org/10.1002/mds.26424] [PMID: 26474316]
[http://dx.doi.org/10.1002/mds.28075] [PMID: 32357266]
[http://dx.doi.org/10.1002/mds.26069] [PMID: 25476529]
[http://dx.doi.org/10.1001/jamaneurol.2021.1335] [PMID: 33999109]
[http://dx.doi.org/10.1016/j.yrtph.2018.05.005] [PMID: 29729297]
[http://dx.doi.org/10.3390/cells11081261] [PMID: 35455941]
[http://dx.doi.org/10.1016/S1474-4422(14)70287-X] [PMID: 25435387]
[http://dx.doi.org/10.1111/ejn.14094] [PMID: 30059179]
[http://dx.doi.org/10.1038/s41582-021-00486-9] [PMID: 33879872]
[http://dx.doi.org/10.1016/j.nbd.2021.105557] [PMID: 34763110]
[http://dx.doi.org/10.1016/j.nbd.2022.105626] [PMID: 35031485]
[http://dx.doi.org/10.3390/jcm10194377] [PMID: 34640395]
[http://dx.doi.org/10.1016/S1474-4422(21)00356-2] [PMID: 34678172]
[http://dx.doi.org/10.1016/j.clineuro.2021.106976] [PMID: 34666273]
[http://dx.doi.org/10.1007/s13311-020-00939-x] [PMID: 33118132]
[http://dx.doi.org/10.3389/fneur.2019.00452] [PMID: 31114542]
[http://dx.doi.org/10.1155/2017/6405278] [PMID: 29081890]
[http://dx.doi.org/10.1016/j.neubiorev.2021.10.019] [PMID: 34688727]
[http://dx.doi.org/10.1177/1545968314562108] [PMID: 25527485]
[http://dx.doi.org/10.1080/15321819.2020.1833917] [PMID: 33078659]
[http://dx.doi.org/10.1016/j.expneurol.2021.113741] [PMID: 33965411]
[http://dx.doi.org/10.1177/0271678X17750351] [PMID: 29271291]
[http://dx.doi.org/10.1002/ana.26291] [PMID: 34951063]
[http://dx.doi.org/10.1155/2020/2410863] [PMID: 32300475]
[http://dx.doi.org/10.14336/AD.2021.0123] [PMID: 34631210]
[http://dx.doi.org/10.3233/JPD-181482] [PMID: 30584163]
[http://dx.doi.org/10.1111/jnc.15516] [PMID: 34532856]
[http://dx.doi.org/10.1007/s11910-015-0571-z] [PMID: 26092313]
[PMID: 23829104]
[http://dx.doi.org/10.1002/mds.27037] [PMID: 28520211]
[http://dx.doi.org/10.1093/brain/awab061] [PMID: 33880502]
[http://dx.doi.org/10.1016/j.nbd.2015.03.005] [PMID: 25771169]
[http://dx.doi.org/10.1177/0023677216653984] [PMID: 27307423]
[http://dx.doi.org/10.3389/fnagi.2022.810860] [PMID: 35296034]
[http://dx.doi.org/10.1523/JNEUROSCI.21-18-07247.2001] [PMID: 11549735]
[http://dx.doi.org/10.1007/s00702-017-1715-x] [PMID: 28357564]
[http://dx.doi.org/10.1093/ilar.48.4.339] [PMID: 17712221]
[http://dx.doi.org/10.1016/S0140-6736(08)60489-4] [PMID: 18374844]
[http://dx.doi.org/10.1080/02688690802448285] [PMID: 19085346]
[http://dx.doi.org/10.1016/j.neubiorev.2007.02.003] [PMID: 17445892]
[http://dx.doi.org/10.3389/fphys.2019.00838] [PMID: 31354509]
[http://dx.doi.org/10.1007/s00429-016-1327-5] [PMID: 27778106]
[http://dx.doi.org/10.1007/s00441-020-03206-9] [PMID: 32356014]
[http://dx.doi.org/10.1186/1471-2164-6-70] [PMID: 15885146]
[http://dx.doi.org/10.1016/j.aanat.2006.09.004] [PMID: 17419547]
[http://dx.doi.org/10.1079/PNS2005452] [PMID: 16313686]
[http://dx.doi.org/10.3389/fgene.2015.00293] [PMID: 26442109]
[http://dx.doi.org/10.1016/S0892-0362(98)00037-3] [PMID: 10192277]
[http://dx.doi.org/10.21307/ane-2017-020] [PMID: 27685773]
[http://dx.doi.org/10.1016/j.neuro.2022.05.006] [PMID: 35569565]
[http://dx.doi.org/10.1002/syn.22060] [PMID: 30009467]
[http://dx.doi.org/10.1016/j.expneurol.2018.02.005] [PMID: 29428213]
[http://dx.doi.org/10.1038/s41598-018-34084-5] [PMID: 30356172]
[http://dx.doi.org/10.1016/j.biochi.2020.10.019] [PMID: 33152422]
[http://dx.doi.org/10.3727/000000002783985314] [PMID: 12588105]
[http://dx.doi.org/10.1177/096368970000900210] [PMID: 10811397]
[http://dx.doi.org/10.1034/j.1600-0404.2001.103005309.x] [PMID: 11328207]
[http://dx.doi.org/10.1016/j.brs.2015.02.003] [PMID: 25758422]
[http://dx.doi.org/10.1177/0271678X17705260] [PMID: 28509598]
[http://dx.doi.org/10.1007/s11307-020-01506-8] [PMID: 32514885]
[http://dx.doi.org/10.1177/0269881119836212] [PMID: 30887871]
[http://dx.doi.org/10.1016/j.brs.2020.03.019] [PMID: 32388196]
[http://dx.doi.org/10.3389/fphar.2022.835827] [PMID: 35370740]
[http://dx.doi.org/10.1038/nprot.2007.393] [PMID: 18007638]
[http://dx.doi.org/10.2174/1871527320666210809120621] [PMID: 35040399]
[http://dx.doi.org/10.4103/1673-5374.335836] [PMID: 35259819]
[http://dx.doi.org/10.1016/j.pbb.2020.173060] [PMID: 33091373]
[http://dx.doi.org/10.1016/j.jns.2022.120220] [PMID: 35313223]
[http://dx.doi.org/10.1177/0271678X20982389] [PMID: 33461410]
[http://dx.doi.org/10.1016/j.nicl.2021.102873] [PMID: 34749290]
[http://dx.doi.org/10.2967/jnumed.121.261939] [PMID: 34272323]
[http://dx.doi.org/10.1007/s00415-021-10685-5] [PMID: 34724571]
[http://dx.doi.org/10.1002/mds.28216] [PMID: 32767618]
[http://dx.doi.org/10.1002/mds.28617] [PMID: 33899255]
[http://dx.doi.org/10.1002/ana.25682] [PMID: 31953875]
[http://dx.doi.org/10.1002/mds.28064] [PMID: 32347983]
[http://dx.doi.org/10.1002/mds.29047] [PMID: 35521899]
[http://dx.doi.org/10.1073/pnas.1812155116] [PMID: 30635412]
[http://dx.doi.org/10.1177/0271678X211004146] [PMID: 33757319]
[http://dx.doi.org/10.1002/mds.28984] [PMID: 35289424]
[http://dx.doi.org/10.1007/s00259-020-05133-x] [PMID: 33369690]
[http://dx.doi.org/10.3389/fnagi.2022.830704] [PMID: 35572127]
[http://dx.doi.org/10.3389/fnins.2022.796129] [PMID: 35401097]
[http://dx.doi.org/10.1097/00001756-200001170-00041] [PMID: 10683860]
[http://dx.doi.org/10.3389/fnagi.2020.599045] [PMID: 33519420]
[http://dx.doi.org/10.1096/fj.06-6092com] [PMID: 17077307]
[http://dx.doi.org/10.2147/NDT.S235562] [PMID: 32184604]
[http://dx.doi.org/10.1016/j.neuroscience.2018.03.026] [PMID: 29592843]
[http://dx.doi.org/10.1016/j.expneurol.2007.10.012] [PMID: 18053987]
[http://dx.doi.org/10.1007/s12031-017-0955-4] [PMID: 28801819]
[http://dx.doi.org/10.1016/j.neuroscience.2013.01.060] [PMID: 23396085]
[http://dx.doi.org/10.1016/0306-4522(94)90605-X] [PMID: 7516500]
[http://dx.doi.org/10.1016/j.nrl.2015.06.011] [PMID: 26304655]
[http://dx.doi.org/10.1016/j.brainresbull.2018.02.010] [PMID: 29462643]
[http://dx.doi.org/10.1111/ejn.13232] [PMID: 26950181]
[http://dx.doi.org/10.1016/j.biopsych.2013.02.015] [PMID: 23541633]
[http://dx.doi.org/10.3389/fnins.2020.590029] [PMID: 33154717]
[http://dx.doi.org/10.1016/j.autneu.2012.04.005] [PMID: 22608184]
[http://dx.doi.org/10.1002/syn.21883] [PMID: 26749375]
[http://dx.doi.org/10.1038/laban0411-119] [PMID: 21427691]
[http://dx.doi.org/10.1212/WNL.35.7.949] [PMID: 3874373]
[http://dx.doi.org/10.1007/s00401-008-0350-x] [PMID: 18273623]
[http://dx.doi.org/10.3389/fnins.2018.00972] [PMID: 30618591]
[http://dx.doi.org/10.1523/JNEUROSCI.2010-15.2016] [PMID: 26843639]
[http://dx.doi.org/10.1159/000334497] [PMID: 22327563]
[http://dx.doi.org/10.2967/jnumed.115.161513] [PMID: 27056614]
[http://dx.doi.org/10.1084/jem.20050163] [PMID: 16129703]
[http://dx.doi.org/10.1016/j.bbr.2020.112607] [PMID: 32199987]
[http://dx.doi.org/10.1016/j.brainres.2019.146521] [PMID: 31697924]
[http://dx.doi.org/10.1016/j.expneurol.2019.02.007] [PMID: 30772369]
[http://dx.doi.org/10.1016/j.brainres.2019.146301] [PMID: 31226324]
[http://dx.doi.org/10.2967/jnumed.121.263039] [PMID: 35177426]
[http://dx.doi.org/10.1016/j.neuroimage.2021.118842] [PMID: 34942366]
[http://dx.doi.org/10.3389/fnsyn.2021.715811] [PMID: 34867258]
[http://dx.doi.org/10.1007/s12149-020-01530-2] [PMID: 32989663]
[http://dx.doi.org/10.1111/jnc.14016] [PMID: 28294334]
[http://dx.doi.org/10.1016/j.nucmedbio.2020.08.002] [PMID: 32861175]
[http://dx.doi.org/10.7150/thno.47585] [PMID: 32724451]
[http://dx.doi.org/10.1002/syn.22077] [PMID: 30368914]
[http://dx.doi.org/10.1186/s12880-019-0375-8] [PMID: 31533645]
[http://dx.doi.org/10.3389/fnins.2019.00799] [PMID: 31417352]
[http://dx.doi.org/10.1038/srep41589] [PMID: 28134302]
[http://dx.doi.org/10.1186/s13550-017-0317-9] [PMID: 28831764]
[http://dx.doi.org/10.1242/dmm.039065] [PMID: 31064773]
[http://dx.doi.org/10.1016/j.neurobiolaging.2017.09.006] [PMID: 29055799]
[http://dx.doi.org/10.1007/s11307-019-01418-2] [PMID: 31392531]
[http://dx.doi.org/10.1016/j.neuroimage.2017.05.066] [PMID: 28583881]
[http://dx.doi.org/10.1016/j.ejphar.2019.172639] [PMID: 31491406]
[http://dx.doi.org/10.3390/molecules23030587] [PMID: 29509680]
[http://dx.doi.org/10.1007/s11307-020-01485-w] [PMID: 32086763]
[http://dx.doi.org/10.1177/0963689718766324] [PMID: 29871515]
[http://dx.doi.org/10.3390/cells8111420] [PMID: 31718058]
[http://dx.doi.org/10.1186/s13287-020-01868-4] [PMID: 32771055]
[http://dx.doi.org/10.3727/096368915X688236] [PMID: 25994923]
[http://dx.doi.org/10.1002/term.2098] [PMID: 26510988]
[http://dx.doi.org/10.1016/j.nbd.2022.105669] [PMID: 35219857]
[http://dx.doi.org/10.1016/j.neurobiolaging.2017.01.010] [PMID: 28189343]
[http://dx.doi.org/10.1016/j.jneumeth.2018.10.037] [PMID: 30391524]
[http://dx.doi.org/10.1371/journal.pone.0202201] [PMID: 30183721]
[http://dx.doi.org/10.2967/jnumed.116.189159] [PMID: 28280215]
[http://dx.doi.org/10.1016/j.nbd.2020.105027] [PMID: 32712266]
[http://dx.doi.org/10.1186/s13550-020-00683-5] [PMID: 32761399]
[http://dx.doi.org/10.1002/mds.27565] [PMID: 30575996]
[http://dx.doi.org/10.1002/mds.27462] [PMID: 30216534]
[http://dx.doi.org/10.1371/journal.pone.0173503] [PMID: 28257461]
[http://dx.doi.org/10.1002/mds.107] [PMID: 30161282]
[http://dx.doi.org/10.1038/s41591-021-01257-1] [PMID: 33649496]
[http://dx.doi.org/10.1038/s41401-019-0313-x] [PMID: 31705124]
[http://dx.doi.org/10.1038/nature23664] [PMID: 28858313]
[http://dx.doi.org/10.1002/stem.1060] [PMID: 22328536]
[http://dx.doi.org/10.3389/fphar.2020.00953] [PMID: 32676027]
[http://dx.doi.org/10.1016/j.omtm.2019.07.002] [PMID: 31406701]
[http://dx.doi.org/10.1016/j.neuroimage.2018.08.016] [PMID: 30102999]
[http://dx.doi.org/10.1038/81834] [PMID: 11100151]
[http://dx.doi.org/10.1016/j.neuroscience.2011.08.041] [PMID: 21884756]
[http://dx.doi.org/10.1016/j.mito.2018.03.001] [PMID: 29563046]
[http://dx.doi.org/10.3390/molecules25071633] [PMID: 32252340]
[PMID: 35631343]
[http://dx.doi.org/10.4061/2011/327089] [PMID: 21603177]
[http://dx.doi.org/10.1007/s10156-012-0544-y] [PMID: 23354935]
[http://dx.doi.org/10.3390/ph15030276] [PMID: 35337075]
[http://dx.doi.org/10.1007/s11307-016-0984-3] [PMID: 27481358]
[http://dx.doi.org/10.1177/0271678X16685105] [PMID: 28079433]
[http://dx.doi.org/10.1007/s11307-018-01313-2] [PMID: 30632003]
[http://dx.doi.org/10.1016/j.nucmedbio.2017.05.011] [PMID: 28719807]
[http://dx.doi.org/10.3389/fphar.2020.00077] [PMID: 32153401]
[http://dx.doi.org/10.1016/j.ejmech.2018.03.035] [PMID: 29604582]
[http://dx.doi.org/10.1177/0269881118788830] [PMID: 30126329]
[http://dx.doi.org/10.1038/s41386-018-0141-6] [PMID: 30026598]
[http://dx.doi.org/10.1016/S0197-4580(02)00065-9] [PMID: 12498954]
[http://dx.doi.org/10.1007/978-3-662-46344-4] [PMID: 26317142]
[http://dx.doi.org/10.1016/S0140-6736(14)61393-3] [PMID: 25904081]
[http://dx.doi.org/10.1016/j.jneumeth.2020.108685] [PMID: 32173400]
[http://dx.doi.org/10.1016/S1474-4422(19)30287-X] [PMID: 31521533]
[http://dx.doi.org/10.1101/cshperspect.a008888] [PMID: 22315721]
[http://dx.doi.org/10.1212/01.wnl.0000277637.33328.d8] [PMID: 17938369]
[http://dx.doi.org/10.3233/JPD-191829] [PMID: 32310186]
[http://dx.doi.org/10.1159/000279653] [PMID: 20413974]
[http://dx.doi.org/10.3390/ijms21144966] [PMID: 32674335]
[http://dx.doi.org/10.1016/j.conb.2021.11.004] [PMID: 34883387]
[http://dx.doi.org/10.1002/mds.27994] [PMID: 32034799]
[http://dx.doi.org/10.1073/pnas.2103425118] [PMID: 34326260]
[http://dx.doi.org/10.1016/j.nbd.2021.105513] [PMID: 34536552]
[http://dx.doi.org/10.1007/s13238-022-00912-8] [PMID: 35334073]
[http://dx.doi.org/10.2174/1566523220666201214115024] [PMID: 33319680]
[http://dx.doi.org/10.1186/s40478-017-0416-x] [PMID: 28143577]
[http://dx.doi.org/10.3233/JPD-140344] [PMID: 25000966]
[http://dx.doi.org/10.3233/JPD-181446] [PMID: 30452424]
[http://dx.doi.org/10.1002/jnr.21032] [PMID: 16941649]
[http://dx.doi.org/10.1038/s41598-020-68874-7] [PMID: 32678262]
[http://dx.doi.org/10.1016/S1474-4422(07)70076-5] [PMID: 17362839]
[http://dx.doi.org/10.1016/S0014-5793(01)03115-5] [PMID: 11734199]
[http://dx.doi.org/10.3233/JPD-2011-11044] [PMID: 22279517]
[http://dx.doi.org/10.1038/33416] [PMID: 9560156]
[http://dx.doi.org/10.1038/26652] [PMID: 9774100]
[PMID: 10914494]
[http://dx.doi.org/10.1038/42166] [PMID: 9278044]
[http://dx.doi.org/10.1016/S0304-3940(00)01701-8] [PMID: 11137760]
[http://dx.doi.org/10.1074/jbc.273.15.8545] [PMID: 9535824]
[http://dx.doi.org/10.1016/j.neulet.2005.09.086] [PMID: 16584840]
[http://dx.doi.org/10.1007/s12149-017-1174-3] [PMID: 28451991]
[http://dx.doi.org/10.1523/JNEUROSCI.23-26-08955.2003] [PMID: 14523098]
[http://dx.doi.org/10.1016/j.expneurol.2013.03.021] [PMID: 23557600]
[http://dx.doi.org/10.1016/j.bbr.2013.12.019] [PMID: 24361083]
[http://dx.doi.org/10.3233/JPD-160921] [PMID: 27802243]
[http://dx.doi.org/10.1007/s00221-017-4962-z] [PMID: 28439627]
[http://dx.doi.org/10.1016/S0074-7742(04)62003-4] [PMID: 15530569]
[http://dx.doi.org/10.1002/ana.20935] [PMID: 16862579]
[http://dx.doi.org/10.1016/j.brainres.2007.06.076] [PMID: 17706185]
[http://dx.doi.org/10.1016/j.brainres.2010.07.060] [PMID: 20678493]
[http://dx.doi.org/10.1002/mds.21306] [PMID: 17230468]
[http://dx.doi.org/10.1073/pnas.0409713102] [PMID: 15716361]
[http://dx.doi.org/10.3389/fnins.2019.00457] [PMID: 31133790]
[http://dx.doi.org/10.1007/s00401-012-0977-5] [PMID: 22491959]
[http://dx.doi.org/10.1016/j.parkreldis.2019.09.025] [PMID: 31621614]
[http://dx.doi.org/10.1038/s41576-019-0205-4] [PMID: 32042148]
[http://dx.doi.org/10.1073/pnas.91.19.8915] [PMID: 8090744]
[http://dx.doi.org/10.1016/j.ymthe.2005.11.015] [PMID: 16413228]
[http://dx.doi.org/10.1089/hum.2011.026] [PMID: 21595499]
[http://dx.doi.org/10.1016/S0166-2236(03)00164-4] [PMID: 12850435]
[http://dx.doi.org/10.1523/JNEUROSCI.22-07-02780.2002] [PMID: 11923443]
[http://dx.doi.org/10.1016/j.nbd.2011.12.013] [PMID: 22182688]
[http://dx.doi.org/10.1186/1750-1326-8-44] [PMID: 24267638]
[http://dx.doi.org/10.1038/s41598-017-06724-9] [PMID: 28743955]
[http://dx.doi.org/10.3390/biomedicines9121876] [PMID: 34944691]
[http://dx.doi.org/10.1038/sj.gt.3300358] [PMID: 9068791]
[http://dx.doi.org/10.1017/S1092852900010075] [PMID: 15744224]
[http://dx.doi.org/10.1093/brain/awl382] [PMID: 17303591]
[http://dx.doi.org/10.1111/ejn.13493] [PMID: 27893183]
[http://dx.doi.org/10.1016/j.expneurol.2009.12.009] [PMID: 20025873]
[http://dx.doi.org/10.1111/cns.12978] [PMID: 29781098]
[http://dx.doi.org/10.1016/j.expneurol.2019.112964] [PMID: 31136763]
[http://dx.doi.org/10.1007/7854_2014_310] [PMID: 24839101]
[http://dx.doi.org/10.3233/JPD-191909] [PMID: 32568105]
[http://dx.doi.org/10.1038/nm1746] [PMID: 18391963]
[http://dx.doi.org/10.1016/S1474-4422(10)70213-1] [PMID: 20846907]
[http://dx.doi.org/10.1016/j.nbd.2015.06.003] [PMID: 26093169]
[http://dx.doi.org/10.1084/jem.20112457] [PMID: 22508839]
[http://dx.doi.org/10.1016/j.nbd.2017.05.014] [PMID: 28576704]
[http://dx.doi.org/10.1016/j.nbd.2019.104525] [PMID: 31276792]
[http://dx.doi.org/10.1186/s40478-017-0494-9] [PMID: 29162163]
[http://dx.doi.org/10.1016/j.nbd.2020.105229] [PMID: 33352233]
[http://dx.doi.org/10.1002/ana.24066] [PMID: 24243558]
[http://dx.doi.org/10.1186/s40478-017-0413-0] [PMID: 28148299]
[http://dx.doi.org/10.3389/fnins.2018.00621] [PMID: 30233303]
[http://dx.doi.org/10.3389/fnins.2022.847074] [PMID: 35368260]
[http://dx.doi.org/10.1016/j.nbd.2021.105599] [PMID: 34952161]
[http://dx.doi.org/10.1007/s00702-002-0808-2] [PMID: 12721813]
[http://dx.doi.org/10.1038/nrneurol.2015.197] [PMID: 26503923]
[http://dx.doi.org/10.1007/s00401-017-1777-8] [PMID: 29039141]
[http://dx.doi.org/10.1371/journal.pone.0008762] [PMID: 20098733]
[http://dx.doi.org/10.1111/j.1749-6632.2009.04365.x] [PMID: 19686202]
[http://dx.doi.org/10.1002/emmm.201302475] [PMID: 23703938]
[http://dx.doi.org/10.1002/ana.24448] [PMID: 26031848]
[http://dx.doi.org/10.1111/j.1365-2990.2008.00937.x] [PMID: 18282157]
[http://dx.doi.org/10.1093/brain/awaa238] [PMID: 32830221]
[http://dx.doi.org/10.1007/s00401-019-02040-w] [PMID: 31254094]
[http://dx.doi.org/10.1016/j.neuron.2019.05.035] [PMID: 31255487]
[http://dx.doi.org/10.1093/brain/awaa096] [PMID: 32380543]
[http://dx.doi.org/10.1186/s13024-018-0257-5] [PMID: 29751824]
[http://dx.doi.org/10.1016/j.neulet.2019.134651] [PMID: 31783082]
[http://dx.doi.org/10.1212/WNL.0000000000003961] [PMID: 28446653]
[http://dx.doi.org/10.3389/fnagi.2022.909273] [PMID: 35966779]
[http://dx.doi.org/10.1038/s41593-020-0589-7] [PMID: 32066981]
[http://dx.doi.org/10.1017/neu.2022.4] [PMID: 35109961]
[http://dx.doi.org/10.1002/ana.21832] [PMID: 19847894]
[http://dx.doi.org/10.1038/466S6a] [PMID: 20739934]
[http://dx.doi.org/10.1016/j.molbrainres.2005.01.012] [PMID: 15790534]
[http://dx.doi.org/10.1111/j.1471-4159.2008.05651.x] [PMID: 18761710]
[http://dx.doi.org/10.1002/ana.26166] [PMID: 34288055]