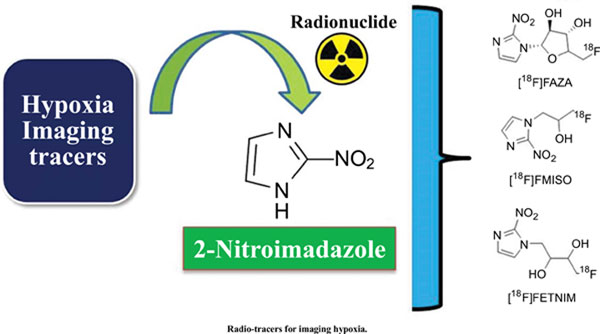
Abstract
The radiolabeled tracers have been extensively utilized to access various physiological and pathological conditions non-invasively, such as cancers, inflammation, and organ-specific imaging. These tracers demonstrate and study tumor hypoxia in several malignancies. Hypoxia is commonly seen in solid tumors. Tumor Hypoxia is a non-physiological condition of reduced oxygen concentration in the tumor. Hypoxia is associated with adverse outcomes such as treatment resistance and metastases in solid tumors. Tumor hypoxia may result in resistance to radiation therapy and chemotherapy, leading to a poor prognosis. It is one of the clinically paramount factors in treatment planning. Various chemical scaffolds are labeled with compatible radioisotopes for imaging hypoxia by Single-photon emission computed tomography (SPECT) and Positron emission tomography (PET). Radionuclides, such as [18F]Flourine, [99mTc]Technetium, [131I]Iodine, [124I] Iodine, and [64Cu]Copper are used for incorporation into different chemical scaffolds.Among them, [18F]Flourine and [64Cu]Copper tagged radiopharmaceuticals are most explored, such as [18F]FMISO, [18F]FAZA, [18F]FETNIM, and N4-methyl thiosemicarbazone [64Cu][Cu (ATSM)]. Some of the promising scaffolds for imaging hypoxia are [18F]EF1, [18F]EF5, [18F]EF3, and [18F]HX4.
This review is focused on developing radiochemistry routes to synthesize different radiopharmaceuticals for imaging hypoxia in clinical and preclinical studies, as described in the literature. The chemist and radiochemist exerted enormous efforts to overcome these obstacles. They have successfully formulated multiple radiopharmaceuticals for hypoxia imaging. Radionuclide incorporation in high selectivity and efficiency (radiochemical yield, specific activity, purity, and radio-scalability) is a need for application perspective. Versatile chemistry, including nucleophilic and electrophilic substitutions, allows the direct or indirect introduction of radioisotopes into molecules of interest. This review will discuss the chemical routes for synthesizing and utilizing different precursors for radiolabeling with radionuclides.We will briefly summaries these radio-labeled tracers' application and biological significance.
Keywords: Radionuclide, Nitroimidazole, Radiotracer, Hypoxia, Diagnostics, Imaging.
[http://dx.doi.org/10.2174/1381612822666160217130049] [PMID: 26898739]
[http://dx.doi.org/10.1200/JCO.2014.60.0759] [PMID: 25800764]
[http://dx.doi.org/10.1634/theoncologist.13-S3-21] [PMID: 18458121]
[http://dx.doi.org/10.1089/ars.2007.1628] [PMID: 17536958]
[http://dx.doi.org/10.1007/s40336-017-0231-1] [PMID: 28596947]
[http://dx.doi.org/10.1007/BF00055376] [PMID: 3552280]
[http://dx.doi.org/10.1634/theoncologist.9-90005-4] [PMID: 15591417]
[http://dx.doi.org/10.1007/978-1-4939-0620-8_3] [PMID: 24729210]
[http://dx.doi.org/10.1089/ars.2013.5378] [PMID: 24512032]
[http://dx.doi.org/10.2967/jnumed.107.045914] [PMID: 18523070]
[http://dx.doi.org/10.1016/j.molonc.2008.03.006] [PMID: 19383328]
[http://dx.doi.org/10.2174/138161212799315849] [PMID: 22272826]
[http://dx.doi.org/10.1259/0007-1285-9-105-606]
[http://dx.doi.org/10.1259/0007-1285-26-312-638] [PMID: 13106296]
[http://dx.doi.org/10.1385/MO:18:4:243] [PMID: 11918451]
[http://dx.doi.org/10.1038/nrc2540] [PMID: 18987634]
[http://dx.doi.org/10.1016/0360-3016(84)90063-4] [PMID: 6231272]
[http://dx.doi.org/10.1038/nrc1367] [PMID: 15170446]
[http://dx.doi.org/10.1016/j.ctro.2019.01.005] [PMID: 30734002]
[http://dx.doi.org/10.3389/fonc.2021.731503]
[http://dx.doi.org/10.1056/NEJM197111182852108] [PMID: 4938153]
[http://dx.doi.org/10.1038/nrc1187] [PMID: 13130303]
[http://dx.doi.org/10.1016/j.arr.2012.10.004] [PMID: 23123177]
[http://dx.doi.org/10.1038/nrc3064] [PMID: 21606941]
[http://dx.doi.org/10.1200/JCO.2000.18.5.1135] [PMID: 10694567]
[http://dx.doi.org/10.1126/science.2479986] [PMID: 2479986]
[http://dx.doi.org/10.2147/vhrm.2006.2.3.213] [PMID: 17326328]
[http://dx.doi.org/10.1016/S0002-9440(10)65550-2] [PMID: 9665470]
[http://dx.doi.org/10.1038/oncsis.2015.14] [PMID: 26029827]
[PMID: 8763890]
[PMID: 22661950]
[http://dx.doi.org/10.1016/j.pharmthera.2016.04.009] [PMID: 27139518]
[http://dx.doi.org/10.1016/S0076-6879(07)35015-5] [PMID: 17998060]
[http://dx.doi.org/10.1016/S0305-7372(03)00003-3] [PMID: 12927570]
[http://dx.doi.org/10.1038/sj.bjc.6605311] [PMID: 19755992]
[PMID: 7727714]
[PMID: 8763846]
[http://dx.doi.org/10.1007/s11307-010-0420-z] [PMID: 20838906]
[http://dx.doi.org/10.1016/S0360-3016(97)00101-6] [PMID: 9226314]
[http://dx.doi.org/10.1006/gyno.1993.1262] [PMID: 8276286]
[http://dx.doi.org/10.1016/S0167-8140(98)00123-6] [PMID: 10368040]
[http://dx.doi.org/10.1148/radiology.166.3.3340773] [PMID: 3340773]
[http://dx.doi.org/10.1358/dnp.2007.20.10.1181355] [PMID: 18301796]
[http://dx.doi.org/10.1126/science.3420417] [PMID: 3420417]
[http://dx.doi.org/10.1021/am9001698] [PMID: 20072726]
[http://dx.doi.org/10.1667/RR0719.1] [PMID: 17390721]
[http://dx.doi.org/10.1056/NEJM197912273012606] [PMID: 229413]
[PMID: 9731480]
[PMID: 10766193]
[http://dx.doi.org/10.1016/S0304-3835(03)00012-0] [PMID: 12767506]
[http://dx.doi.org/10.2307/3580034] [PMID: 10319731]
[http://dx.doi.org/10.1007/978-0-387-85998-9_40] [PMID: 19227481]
[http://dx.doi.org/10.1148/radiol.091669] [PMID: 20032129]
[http://dx.doi.org/10.1002/nbm.716] [PMID: 11746943]
[http://dx.doi.org/10.1080/028418699432536] [PMID: 10606414]
[http://dx.doi.org/10.1038/sj.ki.5000347] [PMID: 16572109]
[http://dx.doi.org/10.1016/j.ijrobp.2008.12.040] [PMID: 19327904]
[http://dx.doi.org/10.1038/sj.ki.5001560] [PMID: 16810286]
[http://dx.doi.org/10.1016/S1476-5586(03)80024-6] [PMID: 14511402]
[http://dx.doi.org/10.1016/S0360-3016(98)00555-0] [PMID: 10348296]
[http://dx.doi.org/10.1007/s10334-004-0083-3] [PMID: 15605277]
[http://dx.doi.org/10.1007/s10555-006-9009-z] [PMID: 17029029]
[http://dx.doi.org/10.2174/0929867053507342] [PMID: 15853714]
[http://dx.doi.org/10.1016/S0360-3016(00)01460-7] [PMID: 11240252]
[http://dx.doi.org/10.2307/3577905] [PMID: 1728059]
[http://dx.doi.org/10.1016/j.radonc.2004.07.018] [PMID: 15588883]
[http://dx.doi.org/10.1038/sj.bjc.6605425] [PMID: 19935799]
[http://dx.doi.org/10.1007/s00259-009-1195-9] [PMID: 19565239]
[http://dx.doi.org/10.2174/1874471011104040361] [PMID: 22202159]
[http://dx.doi.org/10.1007/s10967-019-06649-9]
[http://dx.doi.org/10.1007/s00259-022-05938-y] [PMID: 35994060]
[http://dx.doi.org/10.1161/CIRCIMAGING.119.009791] [PMID: 31910670]
[http://dx.doi.org/10.2967/jnumed.119.230805] [PMID: 31586008]
[http://dx.doi.org/10.1016/S0360-3016(96)00325-2] [PMID: 8892467]
[http://dx.doi.org/10.1007/s00259-007-0424-3] [PMID: 17447061]
[http://dx.doi.org/10.1111/j.1349-7006.2009.01195.x] [PMID: 19459851]
[http://dx.doi.org/10.1016/0883-2889(86)90079-1] [PMID: 3021662]
[PMID: 2738663]
[http://dx.doi.org/10.1016/0969-8043(93)90110-V] [PMID: 8358398]
[http://dx.doi.org/10.1023/A:1018937709835] [PMID: 8008718]
[http://dx.doi.org/10.1016/j.nucmedbio.2005.06.003] [PMID: 16253816]
[http://dx.doi.org/10.1016/j.apradiso.2007.01.005] [PMID: 17379530]
[http://dx.doi.org/10.1007/s10967-013-2753-y]
[http://dx.doi.org/10.1016/j.apradiso.2012.05.022] [PMID: 22871433]
[http://dx.doi.org/10.1016/S0969-8043(02)00185-9] [PMID: 12433044]
[PMID: 15632040]
[http://dx.doi.org/10.1007/s00259-009-1154-5] [PMID: 19430784]
[http://dx.doi.org/10.1016/S0969-8051(02)00442-0] [PMID: 12745023]
[http://dx.doi.org/10.1002/(SICI)1099-1344(199901)42:1<3:AID-JLCR160>3.0.CO;2-H]
[http://dx.doi.org/10.1016/S0040-4039(01)00099-5]
[http://dx.doi.org/10.1016/j.apradiso.2004.12.004] [PMID: 15799867]
[http://dx.doi.org/10.1016/j.apradiso.2010.04.011] [PMID: 20478712]
[http://dx.doi.org/10.1016/j.apradiso.2011.02.025] [PMID: 21420304]
[http://dx.doi.org/10.1016/j.nucmedbio.2010.09.002] [PMID: 21315279]
[http://dx.doi.org/10.1186/s13550-020-00621-5] [PMID: 32274601]
[http://dx.doi.org/10.1039/b705989b] [PMID: 17992274]
[http://dx.doi.org/10.1016/j.ica.2010.01.038]
[http://dx.doi.org/10.1016/j.nucmedbio.2011.08.008] [PMID: 22033022]
[PMID: 34954786]
[http://dx.doi.org/10.1524/ract.93.4.239.64070]
[http://dx.doi.org/10.1002/jlcr.3973] [PMID: 35466453]
[http://dx.doi.org/10.1016/S0969-8043(99)00096-2] [PMID: 10581679]
[http://dx.doi.org/10.1016/S0969-8043(00)00102-0] [PMID: 11144255]
[http://dx.doi.org/10.1007/s00259-004-1573-2] [PMID: 15197503]
[http://dx.doi.org/10.1007/s00259-010-1437-x] [PMID: 20369236]
[http://dx.doi.org/10.1073/pnas.1102526108] [PMID: 21873245]
[http://dx.doi.org/10.1016/j.radonc.2013.08.031] [PMID: 24044790]
[http://dx.doi.org/10.1016/j.ijrobp.2014.09.045] [PMID: 25491505]
[http://dx.doi.org/10.1016/j.nucmedbio.2014.12.015] [PMID: 25725983]
[http://dx.doi.org/10.1148/radiology.194.3.7862981] [PMID: 7862981]
[PMID: 11535732]
[http://dx.doi.org/10.1016/j.bmc.2011.02.041] [PMID: 21419635]
[http://dx.doi.org/10.1007/s12149-020-01563-7] [PMID: 33400147]
[http://dx.doi.org/10.1002/jmrs.603] [PMID: 35760568]