Abstract
Background: Several genetic alterations in cell growth regulatory genes, such as BRAF, are associated with colorectal cancer. Due to the introduction of biological agents designed to treat cancer, diagnostic tests using nucleic acids extracted from formalin-fixed and paraffin-embedded tissues are becoming more common.
Objective: This study aimed to determine the incidence of BRAF mutations in colorectal cancer patients.
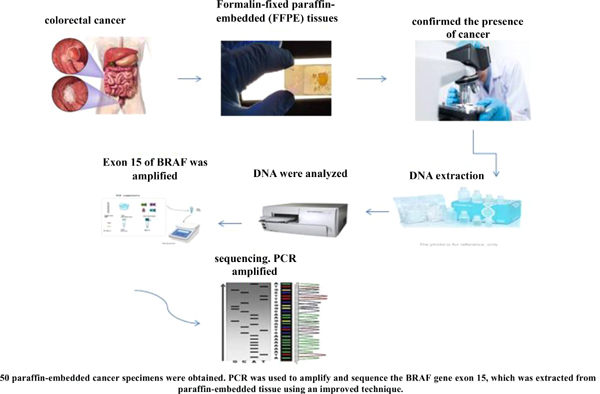
Materials and Methods: 50 paraffin-embedded cancer specimens were obtained from Imam Reza Hospital of Birjand in Iran. PCR was used to amplify and sequence the BRAF gene exon 15, which was extracted from paraffin-embedded tissue using an improved technique.
Results: 2/43 (4%) of patients with colorectal cancer exhibited the BEAF V600E mutation. Most of the mutations occurred in patients over 50 years of age.
Conclusion: To understand how genetics and environment interact to influence the low incidence of BRAF mutations in the east of Iran, further research is needed to determine what is driving this low incidence of BRAF mutations and what factors contribute to it.
Keywords: Colorectal cancer, BRAF mutation, PCR, gene, DNA, cancer.