Abstract
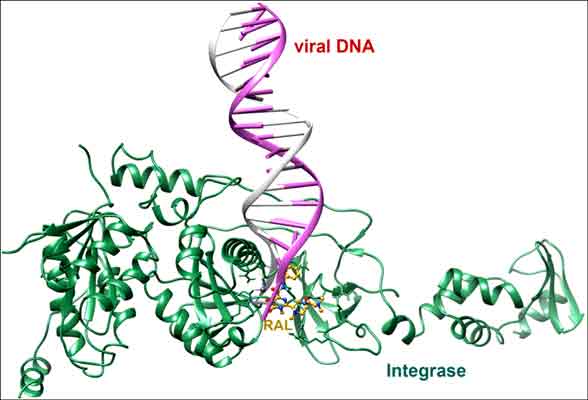
Human immunodeficiency virus type 1 (HIV-1) integrase (IN) is an essential enzyme in the viral replication cycle as it catalyzes the insertion of the reverse transcribed viral DNA into host chromosome. The structure of prototype foamy virus (PFV) IN has structural and functional homology with HIV-1 IN (no full-length structure available). In this study, we have used PFV IN-DNA complex as a surrogate model for HIV-1 IN-DNA complex to investigate the binding modes of N-methyl pyrimidones (NMPs) by QMpolarized ligand docking (QPLD), binding free energy calculations and molecular dynamics simulations. The O,O,O donor atom triad of NMPs show metal chelation with divalent Mg2+ ions in the active site of PFV IN, in perfect agreement with the proposed mechanism of IN strand transfer inhibitors (INSTIs). The results also show that the benzyl group of compounds fit into a pocket to displace the 3'-terminal adenosine of viral DNA from the IN active site making it unavailable for the nucleophile to attack the target DNA in the strand transfer (ST) reaction. The halobenzyl moiety show hydrophobic interactions with conserved PFV IN Tyr212 and Pro214 residues, corresponding to HIV-1 IN Tyr143 and Pro145, respectively. Molecular dynamics (MD) simulations gave important insights into the structural and chemical basis involved in ST inhibition. Based on MD results, hydrogen bond with Tyr212, coordinate bonds with Mg2+ ions, and hydrophobic interactions play an important role in the stabilization of compounds. Our results provide additional insight into the possible mechanism of action and binding mode of NMPs, and might have implications for rational design of specific HIV-1 INSTIs with improved affinity and selectivity.
Keywords: HIV-1, integrase, Metal chelation, MD simulations, QPLD, Strand transfer.