Abstract
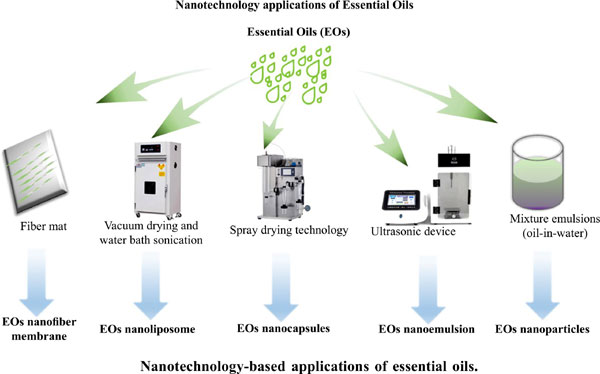
Essential oils (EOs), which are volatile aromatic substances extracted from plants, exhibit antibacterial, antitumor, antiviral, antioxidant, anti-inflammatory, and other effects. Eos are widely used in different fields because of their various biological activities. EOs are volatile and insoluble in water, so their effective utilization rate is greatly reduced. In this regard, researchers propose to use nanotechnology to construct an EOs nanosystem to solve the application problems and improve the utilization rate of EOs. This review summarizes the latest research progress and application status of EOs nanocapsules, EOs nanoemulsion, EOs nanofiber membrane, EOs nanoparticles and EOs nanoliposome, including the methodologies, characteristics and applications.Analyzes the advantages and disadvantages of existing EOs nanotechnology and provides an outlook for future development.
Keywords: Essential oils, nanotechnology, nanocapsule, nanoemulsion, nanofibers, nanoparticles, nanoliposome.
[http://dx.doi.org/10.1016/j.ijbiomac.2022.01.066] [PMID: 35041880]
[http://dx.doi.org/10.1177/1934578X0800300706]
[http://dx.doi.org/10.3390/molecules28031176] [PMID: 36770842]
[http://dx.doi.org/10.1021/acs.jafc.5b01883] [PMID: 26118760]
[http://dx.doi.org/10.3390/molecules25225482] [PMID: 33238598]
[http://dx.doi.org/10.1002/ffj.3328]
[http://dx.doi.org/10.1016/j.ijpharm.2016.01.060] [PMID: 26827919]
[http://dx.doi.org/10.3892/ijo.2018.4314] [PMID: 29568932]
[http://dx.doi.org/10.1016/j.indcrop.2015.09.048]
[http://dx.doi.org/10.1016/j.indcrop.2018.09.048]
[http://dx.doi.org/10.1016/j.foodcont.2015.05.032]
[http://dx.doi.org/10.1016/j.foodcont.2015.01.045]
[http://dx.doi.org/10.1111/jam.12453] [PMID: 24779581]
[http://dx.doi.org/10.1016/j.antiviral.2010.11.010] [PMID: 21095205]
[http://dx.doi.org/10.1016/j.indcrop.2017.12.071]
[http://dx.doi.org/10.1055/s-0035-1545842] [PMID: 25856436]
[http://dx.doi.org/10.1016/j.lwt.2016.01.017]
[http://dx.doi.org/10.1016/j.indcrop.2012.12.022]
[http://dx.doi.org/10.1016/j.jep.2017.10.013] [PMID: 29037915]
[http://dx.doi.org/10.1016/j.jep.2021.114136] [PMID: 33892069]
[http://dx.doi.org/10.1080/10717544.2021.1905748] [PMID: 33787431]
[http://dx.doi.org/10.1016/j.foodchem.2015.01.115]
[http://dx.doi.org/10.1016/j.colsurfb.2020.110784] [PMID: 31935631]
[http://dx.doi.org/10.1016/j.msec.2020.111783] [PMID: 33545910]
[http://dx.doi.org/10.1080/21691401.2019.1582539] [PMID: 30857435]
[http://dx.doi.org/10.1016/j.ijbiomac.2018.12.161] [PMID: 30593811]
[http://dx.doi.org/10.2147/IJN.S157019] [PMID: 29588587]
[http://dx.doi.org/10.2147/IJN.S99317] [PMID: 26955273]
[http://dx.doi.org/10.1016/j.msec.2018.06.013] [PMID: 30033312]
[http://dx.doi.org/10.1016/j.ijpharm.2010.10.055] [PMID: 21056643]
[http://dx.doi.org/10.1016/j.ijbiomac.2018.10.144] [PMID: 30352231]
[http://dx.doi.org/10.3390/ma11010122] [PMID: 29342838]
[http://dx.doi.org/10.1016/j.colsurfb.2018.08.069] [PMID: 30199764]
[http://dx.doi.org/10.1016/j.fitote.2019.104195] [PMID: 31175953]
[http://dx.doi.org/10.1016/j.foodhyd.2013.06.003]
[http://dx.doi.org/10.1016/j.foodres.2018.02.053]
[http://dx.doi.org/10.1007/s12274-017-1908-5]
[http://dx.doi.org/10.1016/j.indcrop.2018.05.058]
[http://dx.doi.org/10.1016/j.colsurfb.2013.08.038] [PMID: 24077112]
[http://dx.doi.org/10.1039/C6PY01782G]
[http://dx.doi.org/10.1016/j.progpolymsci.2016.06.004]
[http://dx.doi.org/10.1016/j.foodres.2007.07.004]
[http://dx.doi.org/10.1016/j.ijpharm.2015.11.042] [PMID: 26631640]
[http://dx.doi.org/10.1016/j.foodchem.2018.06.140] [PMID: 30100436]
[http://dx.doi.org/10.1016/j.foodcont.2018.07.009]
[PMID: 29389592]
[http://dx.doi.org/10.1016/j.foodres.2018.05.066] [PMID: 30007714]
[http://dx.doi.org/10.1016/j.jbiotec.2016.07.005] [PMID: 27416793]
[http://dx.doi.org/10.1016/j.foodres.2016.01.033]
[http://dx.doi.org/10.1016/j.biopha.2017.04.020] [PMID: 28437891]
[http://dx.doi.org/10.1080/10408398.2015.1006767] [PMID: 26114624]
[http://dx.doi.org/10.1016/j.jfoodeng.2014.09.003]
[http://dx.doi.org/10.1016/j.lwt.2016.08.046]
[http://dx.doi.org/10.1007/s11356-018-2068-1]
[http://dx.doi.org/10.1016/j.foodcont.2017.01.001]
[http://dx.doi.org/10.1016/j.ecoenv.2017.04.041] [PMID: 28456128]
[http://dx.doi.org/10.1002/pi.2395]
[http://dx.doi.org/10.1016/j.foodchem.2013.11.037] [PMID: 24423542]
[http://dx.doi.org/10.1515/ijfe-2015-0145]
[http://dx.doi.org/10.1016/j.colsurfb.2016.12.035] [PMID: 28064095]
[http://dx.doi.org/10.2147/IJN.S99317] [PMID: 26955273]
[http://dx.doi.org/10.1016/j.envres.2015.09.024] [PMID: 26615225]
[http://dx.doi.org/10.1080/10643389.2015.1109913]
[http://dx.doi.org/10.1016/j.carbpol.2017.09.043] [PMID: 29050578]
[http://dx.doi.org/10.1016/j.carbpol.2015.11.073] [PMID: 26794956]
[http://dx.doi.org/10.1016/j.foodchem.2017.03.113] [PMID: 28449997]
[http://dx.doi.org/10.1016/j.indcrop.2018.08.001]
[http://dx.doi.org/10.1016/j.ijfoodmicro.2018.12.007] [PMID: 30553179]
[http://dx.doi.org/10.1002/smll.200400130] [PMID: 17193427]
[http://dx.doi.org/10.1016/j.envpol.2012.08.011] [PMID: 22995930]
[http://dx.doi.org/10.1016/j.jksus.2017.06.012]
[http://dx.doi.org/10.1016/j.carbpol.2015.10.093] [PMID: 26686150]
[http://dx.doi.org/10.1016/j.ijbiomac.2017.05.063] [PMID: 28526346]
[http://dx.doi.org/10.1002/jsfa.9050] [PMID: 29635845]
[http://dx.doi.org/10.1016/j.ijbiomac.2015.08.010] [PMID: 26257380]
[http://dx.doi.org/10.1016/j.ijbiomac.2017.04.002] [PMID: 28380334]
[http://dx.doi.org/10.1016/j.lwt.2018.06.013]
[http://dx.doi.org/10.1016/j.ijpharm.2017.01.013] [PMID: 28089935]
[http://dx.doi.org/10.1016/j.lwt.2016.02.037]
[http://dx.doi.org/10.1016/j.foodchem.2018.09.085] [PMID: 30724177]
[http://dx.doi.org/10.1016/j.carbpol.2015.11.053] [PMID: 26794740]
[http://dx.doi.org/10.2174/1389557516666160801111507] [PMID: 27488582]
[http://dx.doi.org/10.1016/j.tifs.2018.07.009]
[http://dx.doi.org/10.1016/j.nano.2015.09.004] [PMID: 26410277]
[http://dx.doi.org/10.1016/j.jddst.2017.06.013]
[http://dx.doi.org/10.1016/j.foodres.2016.06.022] [PMID: 29606252]
[http://dx.doi.org/10.1080/00914037.2017.1332623]
[http://dx.doi.org/10.3109/08982104.2013.819888] [PMID: 23879218]
[http://dx.doi.org/10.1016/j.foodcont.2015.09.034]
[http://dx.doi.org/10.1016/j.foodcont.2018.03.047]
[http://dx.doi.org/10.1111/jfs.12271]
[http://dx.doi.org/10.31080/ASMI.2020.04.0768]
[http://dx.doi.org/10.1016/j.cherd.2010.01.020]