Abstract
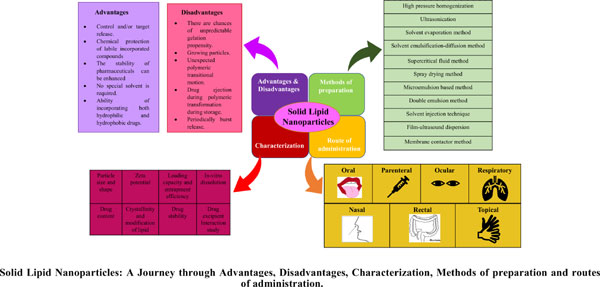
Solid lipid nanoparticles (SLN) have several potential uses in research for medicine such as drug discovery and drug delivery, an area at the forefront of evolving area of nanobiotechnology. In general, SLNs were created to address the drawbacks of conventional colloidal carriers, including emulsions, liposomes, and polymeric nanoparticles since they provide various advantages such as favourable release profiles and tailored drug delivery with outstanding physical-chemical stability. Solid lipid nanoparticles are spherical solid lipid particles that are distributed in water or an aqueous surfactant solution and are in the nanometer size range. Therefore, SLN is used to deliver hydrophilic and lipophilic drugs. The review article focuses on various aspects of SLN including the structure, the influence of excipients, the drug incorporation model, the principle of release, the method of preparation, characterization, the route of administration and biodistribution, and the application of SLN.
Keywords: Nanoparticles, nanotechnology, solid lipid nanoparticles, colloidal drug carrier, drug incorporation, applications.
[http://dx.doi.org/10.13040/IJPSR.0975-8232.10]
[http://dx.doi.org/10.1155/2012/750891] [PMID: 22175030]
[http://dx.doi.org/10.1358/mf.2005.27.2.876286] [PMID: 15834465]
[http://dx.doi.org/10.13040/IJPSR.0975-8232.9]
[http://dx.doi.org/10.13040/IJPSR.0975-8232.5]
[http://dx.doi.org/10.13040/IJPSR.0975-8232.6]
[http://dx.doi.org/10.1208/s12249-010-9563-0] [PMID: 21174180]
[http://dx.doi.org/10.23893/1307-2080.APS.05616]
[http://dx.doi.org/10.1016/j.chemphyslip.2020.104988] [PMID: 33035545]
[http://dx.doi.org/10.1208/s12249-019-1301-7] [PMID: 30721373]
[PMID: 34400962]
[http://dx.doi.org/10.1080/03639045.2018.1483385] [PMID: 29874944]
[http://dx.doi.org/10.1080/03639045.2019.1569023] [PMID: 30632399]
[http://dx.doi.org/10.1080/21691401.2019.1593858] [PMID: 30942627]
[http://dx.doi.org/10.1016/j.foodchem.2017.11.091] [PMID: 29329873]
[http://dx.doi.org/10.1186/s40360-021-00523-9] [PMID: 34587996]
[http://dx.doi.org/10.1166/jnn.2015.9184] [PMID: 26328443]
[http://dx.doi.org/10.1186/s12941-019-0333-x] [PMID: 31706304]
[http://dx.doi.org/10.1080/21691401.2017.1307207] [PMID: 28368657]
[http://dx.doi.org/10.1155/2018/3021738] [PMID: 29854465]
[http://dx.doi.org/10.4274/tjps.galenos.2018.82160] [PMID: 32454745]
[http://dx.doi.org/10.15171/apb.2016.04]
[http://dx.doi.org/10.4103/0975-1483.83765] [PMID: 21897661]
[PMID: 19746839]
[http://dx.doi.org/10.1111/j.2042-7158.2010.01225.x] [PMID: 21749381]
[PMID: 21556343]
[PMID: 26430454]
[http://dx.doi.org/10.3109/13880209.2014.991836] [PMID: 25853953]
[http://dx.doi.org/10.1016/j.ijpharm.2006.11.004] [PMID: 17161566]
[http://dx.doi.org/10.1021/mp4006968] [PMID: 24621456]
[http://dx.doi.org/10.1016/j.biopha.2021.111354] [PMID: 33561642]
[http://dx.doi.org/10.1016/j.colsurfb.2014.01.055] [PMID: 24607519]
[http://dx.doi.org/10.1002/jps.22435] [PMID: 21491449]
[http://dx.doi.org/10.1016/j.colsurfb.2012.04.027] [PMID: 22609590]
[PMID: 24379671]
[http://dx.doi.org/10.3109/10717544.2014.882446] [PMID: 24512431]
[http://dx.doi.org/10.3109/08982100903443065] [PMID: 19958118]
[http://dx.doi.org/10.15171/apb.2016.032] [PMID: 27478786]
[http://dx.doi.org/10.1007/s13204-017-0547-1]
[http://dx.doi.org/10.3109/03639045.2013.810636] [PMID: 23826860]
[http://dx.doi.org/10.1016/j.ejpb.2011.06.005] [PMID: 21726641]
[http://dx.doi.org/10.1016/S2221-6189(13)60144-4]
[PMID: 18203440]
[http://dx.doi.org/10.1016/S0142-9612(02)00578-1] [PMID: 12593960]
[http://dx.doi.org/10.22270/jddt.v8i6.2033]
[http://dx.doi.org/10.13040/IJPSR.0975-8232.13]
[http://dx.doi.org/10.1016/j.ijpharm.2006.12.043] [PMID: 17287099]
[http://dx.doi.org/10.1016/j.ijpharm.2021.120428] [PMID: 33662465]
[http://dx.doi.org/10.1590/s2175-97902017000115012]
[http://dx.doi.org/10.2147/IJN.S100625] [PMID: 26869787]
[http://dx.doi.org/10.1371/journal.pone.0203405] [PMID: 30161251]
[http://dx.doi.org/10.1080/10717544.2017.1413444] [PMID: 29239242]
[http://dx.doi.org/10.17795/jjnpp-33968]
[http://dx.doi.org/10.5530/ijper.53.2s.52]
[http://dx.doi.org/10.35333/jrp.2020.203]
[http://dx.doi.org/10.2147/IJN.S247935] [PMID: 32440115]
[http://dx.doi.org/10.1186/s12951-020-00604-7] [PMID: 32164731]
[http://dx.doi.org/10.37275/nasetjournal.v2i2.21]
[http://dx.doi.org/10.4314/tjpr.v16i9.4]
[http://dx.doi.org/10.5530/ijpi.2020.3.59]
[http://dx.doi.org/10.1186/1476-511X-11-72] [PMID: 22695222]
[http://dx.doi.org/10.3390/pharmaceutics14020409] [PMID: 35214141]
[http://dx.doi.org/10.3390/pharmaceutics14071393] [PMID: 35890289]
[http://dx.doi.org/10.3390/pharmaceutics13040523] [PMID: 33918870]
[http://dx.doi.org/10.34172/apb.2021.010] [PMID: 33747856]
[http://dx.doi.org/10.2147/IJN.S335482] [PMID: 34853513]
[http://dx.doi.org/10.1039/D1RA07638H] [PMID: 35424603]
[http://dx.doi.org/10.1007/s13346-020-00839-9] [PMID: 32804301]
[http://dx.doi.org/10.1016/j.chemphyslip.2021.105123] [PMID: 34403685]
[http://dx.doi.org/10.2217/nnm-2020-0192] [PMID: 32812483]
[http://dx.doi.org/10.2147/DDDT.S102500] [PMID: 28684900]
[http://dx.doi.org/10.1111/jocd.12470] [PMID: 29226503]
[http://dx.doi.org/10.3390/nano8030159] [PMID: 29533979]
[http://dx.doi.org/10.2478/v10007-012-0040-z] [PMID: 23333884]