Abstract
Background: Indazole is a heterocyclic motif widely used in medicinal chemistry due to its positive photophysical properties. The development of new methods for synthesizing the indazole scaffold is of great importance in drug discovery.
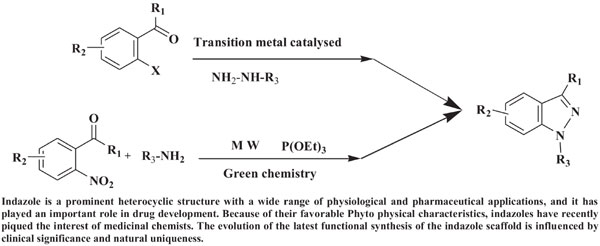
Methods: This study presents a detailed review of current advances in indazole synthesis, focusing on catalyst-based and green chemistry approaches. The analysis is classified based on acid-base and transition-metal catalysts and green chemistry methods. Catalyst-based advances have given a new impetus to the synthesis of this effective pharmacophore.
Results: The extensive literature on indazole synthesis demonstrates the notable progress achieved through catalyst-based approaches. These methods have enabled researchers to create a wide range of indazole derivatives and analogs, facilitating their application in pharmaceutical products and organic molecules. The use of acid-base and transition-metal catalysts has been particularly effective in enhancing the efficiency and selectivity of indazole synthesis.
Conclusion: Indazoles and their variants are widely used in pharmaceutical products and organic molecules. The recent literature indicates that catalyst-based approaches have resulted in significant advancements in indazole synthesis. This review may be useful for researchers in medicinal chemistry, content chemistry, and agrochemistry.
Keywords: 1H-indazole, 2H-indazole, transition metal catalyst, acid-base catalyst, green chemistry, indazole synthesis.
[http://dx.doi.org/10.1016/j.jinorgbio.2012.04.001] [PMID: 22687494]
[http://dx.doi.org/10.1016/S0065-2725(03)85002-X]
[http://dx.doi.org/10.1021/ci600549q] [PMID: 17824684]
[http://dx.doi.org/10.1016/j.bmc.2015.02.004] [PMID: 25771484]
[http://dx.doi.org/10.1016/j.bmcl.2015.09.074] [PMID: 26463130]
[http://dx.doi.org/10.1073/pnas.1411294111] [PMID: 25453074]
[http://dx.doi.org/10.1021/acs.jmedchem.5b00857] [PMID: 26551970]
[http://dx.doi.org/10.1177/0091270009335768] [PMID: 19398602]
[http://dx.doi.org/10.1016/j.bmcl.2016.01.037] [PMID: 26810260]
[http://dx.doi.org/10.1111/jphp.12440] [PMID: 26076716]
[http://dx.doi.org/10.1039/C5OB00778J] [PMID: 26080733]
[http://dx.doi.org/10.1021/acsmedchemlett.5b00322] [PMID: 26713102]
[http://dx.doi.org/10.1021/acs.jmedchem.5b01208]
[http://dx.doi.org/10.1016/j.ejmech.2015.09.034] [PMID: 26451772]
[http://dx.doi.org/10.1021/acs.jmedchem.6b01626] [PMID: 28225269]
[http://dx.doi.org/10.3390/molecules22111864] [PMID: 29088121]
[http://dx.doi.org/10.1021/jm0302039] [PMID: 14667220]
[http://dx.doi.org/10.1016/j.bmcl.2013.03.075] [PMID: 23566519]
[http://dx.doi.org/10.1021/jm800112s] [PMID: 18517187]
[http://dx.doi.org/10.1016/j.ejmech.2017.10.008] [PMID: 29032031]
[http://dx.doi.org/10.1111/cbdd.12984] [PMID: 28338292]
[http://dx.doi.org/10.1016/j.bmcl.2013.02.034] [PMID: 23454017]
[http://dx.doi.org/10.1007/s40265-017-0752-y] [PMID: 28474297]
[http://dx.doi.org/10.1016/j.tetlet.2013.01.030]
[http://dx.doi.org/10.1007/s11164-015-2379-5]
[http://dx.doi.org/10.1111/j.1476-5381.1996.tb15594.x] [PMID: 8842434]
[http://dx.doi.org/10.1042/BCJ20160068] [PMID: 27234586]
[http://dx.doi.org/10.4137/CMO.S10594]
[http://dx.doi.org/10.1634/theoncologist.2012-0055] [PMID: 22733795]
[http://dx.doi.org/10.5772/intechopen]
[http://dx.doi.org/10.1093/jnci/95.7.516] [PMID: 12671019]
(b) Bartsch, R.A.; Yang, I.W. Phase transfer catalyzed synthesis of indazoles from o -alkylbenzenediazonium tetrafluoroborates. J. Heterocycl. Chem., 1984, 21(4), 1063-1064.
[http://dx.doi.org/10.1002/jhet.5570210428];
c) Sun, J.H.; Teleha, C.A.; Yan, J.S.; Rodgers, J.D.; Nugiel, D.A. Efficient synthesis of 5-(bromomethyl)- and 5-(aminomethyl)-1-THP indazole. J. Org. Chem., 1997, 62(16), 5627-5629.
[http://dx.doi.org/10.1021/jo970375b];
d) Souers, A.J.; Gao, J.; Brune, M.; Bush, E.; Wodka, D.; Vasudevan, A.; Judd, A.S.; Mulhern, M.; Brodjian, S.; Dayton, B.; Shapiro, R.; Hernandez, L.E.; Marsh, K.C.; Sham, H.L.; Collins, C.A.; Kym, P.R. Identification of 2-(4-benzyloxyphenyl)-N- [1-(2-pyrrolidin-1-yl ethyl)-1H-indazol-6-yl]acetamide, an orally efficacious melanin-concentrating hormone receptor 1 antagonist for the treatment of obesity. J. Med. Chem., 2005, 48(5), 1318-1321.
[http://dx.doi.org/10.1021/jm0490890] [PMID: 15743174]
[http://dx.doi.org/10.1023/A:1026008821698];
b) Lokhande, P.D.; Raheem, A.; Sabale, S.T.; Chabukswar, A.R.; Jagdale, S.C. An efficient synthesis of 1-H indazoles. Tetrahedron Lett., 2007, 48(39), 6890-6892.
[http://dx.doi.org/10.1016/j.tetlet.2007.07.162];
c) Counceller, C.M.; Eichman, C.C.; Wray, B.C.; Stambuli, J.P. A practical, metal-free synthesis of 1H-indazoles. Org. Lett., 2008, 10(5), 1021-1023.
[http://dx.doi.org/10.1021/ol800053f] [PMID: 18229936]
[http://dx.doi.org/10.1002/anie.200700101] [PMID: 17385767];
b) Liu, Z.; Shi, F.; Martinez, P.D.G.; Raminelli, C.; Larock, R.C. Synthesis of indazoles by the [3+2] cycloaddition of diazo compounds with arynes and subsequent acyl migration. J. Org. Chem., 2008, 73(1), 219-226.
[http://dx.doi.org/10.1021/jo702062n] [PMID: 18067316];
c) Spiteri, C.; Keeling, S.; Moses, J.E. New synthesis of 1-substituted-1H-indazoles via 1,3-dipolar cycloaddition of in situ generated nitrile imines and benzyne. Org. Lett., 2010, 12(15), 3368-3371.
[http://dx.doi.org/10.1021/ol101150t] [PMID: 20608661];
d) Wu, C.; Fang, Y.; Larock, R.C.; Shi, F. Synthesis of 2H-indazoles by the [3 + 2] cycloaddition of arynes and sydnones. Org. Lett., 2010, 12(10), 2234-2237.
[http://dx.doi.org/10.1021/ol100586r] [PMID: 20394430];
e) Li, P.; Zhao, J.; Wu, C.; Larock, R.C.; Shi, F. Synthesis of 3-substituted indazoles from arynes and N-tosylhydrazones. Org. Lett., 2011, 13(13), 3340-3343.
[http://dx.doi.org/10.1021/ol201086g] [PMID: 21630698];
f) Chen, G.; Hu, M.; Peng, Y. Switchable synthesis of 3-Substituted 1 H -indazoles and 3,3-disubstituted 3 H -indazole-3-phosphonates tuned by phosphoryl groups. J. Org. Chem., 2018, 83(3), 1591-1597.
[http://dx.doi.org/10.1021/acs.joc.7b02857] [PMID: 29283256]
[http://dx.doi.org/10.1039/c1cs15082k] [PMID: 21643614]
[http://dx.doi.org/10.1002/ajoc.202000300]
[http://dx.doi.org/10.1016/j.trac.2018.10.022]
[http://dx.doi.org/10.1016/j.jsps.2018.07.011] [PMID: 30627046]
[http://dx.doi.org/10.1021/ol062890e] [PMID: 17249803]
[http://dx.doi.org/10.1002/aoc.2956]
[http://dx.doi.org/10.1080/00397911.2011.614714]
[http://dx.doi.org/10.3390/molecules23030674] [PMID: 29547568]
[http://dx.doi.org/10.1021/ol300847v] [PMID: 22545771]
[http://dx.doi.org/10.1039/C1OB05875D] [PMID: 22183249]
[http://dx.doi.org/10.1016/j.tetlet.2005.08.143]
[http://dx.doi.org/10.1021/jo049658b] [PMID: 15307726]
[http://dx.doi.org/10.1021/acs.orglett.6b00611] [PMID: 26990834]
[http://dx.doi.org/10.1002/ejoc.201701149]
[http://dx.doi.org/10.1039/C4QO00244J]
[http://dx.doi.org/10.1021/acs.orglett.8b03564] [PMID: 30576157]
[http://dx.doi.org/10.1002/adsc.201500953]
[http://dx.doi.org/10.1039/C3RA45298K]
[http://dx.doi.org/10.1021/ol201409j] [PMID: 21644532]
[http://dx.doi.org/10.1055/s-0033-1340979]
[http://dx.doi.org/10.1139/cjc-2018-0428]
[http://dx.doi.org/10.1039/C2CY20590D]
[http://dx.doi.org/10.1039/c1cc13908h] [PMID: 21826369]
[http://dx.doi.org/10.1039/C7OB02323E] [PMID: 28953279]
[http://dx.doi.org/10.9767/bcrec.13.1.963.82-88]
[http://dx.doi.org/10.1002/chem.201404506] [PMID: 25224915]
[http://dx.doi.org/10.1021/ja4033555] [PMID: 23711098]
[http://dx.doi.org/10.1002/anie.201602224] [PMID: 27121133]
[http://dx.doi.org/10.1021/ol500865j] [PMID: 24754303]
[http://dx.doi.org/10.1021/acs.orglett.6b00727] [PMID: 27082502]
[http://dx.doi.org/10.1021/ja402761p] [PMID: 23642256]
[http://dx.doi.org/10.1021/acs.joc.6b02548] [PMID: 27936695]
[http://dx.doi.org/10.1021/acs.orglett.7b00631] [PMID: 28514173];
(b) Long, Z.; Yang, Y.; You, J. Rh (III)-Catalyzed [4 + 1]-annulation of azoxy compounds with alkynes: A regioselective approach to 2H-indazoles. Org. Lett., 2017, 19(11), 2781-2784.
[http://dx.doi.org/10.1021/acs.orglett.7b00982] [PMID: 28514177]
[http://dx.doi.org/10.1021/acs.joc.8b00501] [PMID: 29547287]
[http://dx.doi.org/10.1021/acs.orglett.5b03368] [PMID: 26741169]
[http://dx.doi.org/10.1021/acs.orglett.8b03488] [PMID: 30618258]
[http://dx.doi.org/10.1039/C6SC03888C] [PMID: 28616139]
[http://dx.doi.org/10.1039/c0ob00021c] [PMID: 20835450]
[http://dx.doi.org/10.1039/b312154m] [PMID: 14737353]
[http://dx.doi.org/10.1021/acs.orglett.7b00410] [PMID: 28271896]
[http://dx.doi.org/10.1021/acs.orglett.5b00765] [PMID: 26154712]
[http://dx.doi.org/10.1021/jo048671t] [PMID: 15651807]
[http://dx.doi.org/10.1002/anie.200902323] [PMID: 19681082]
[http://dx.doi.org/10.1021/jo8017236] [PMID: 18947186]
[http://dx.doi.org/10.1021/acs.orglett.5b02136] [PMID: 26308587]
[http://dx.doi.org/10.1021/ol990409x] [PMID: 10814366]
[http://dx.doi.org/10.1021/jo00091a026]
[http://dx.doi.org/10.1021/ol902537d] [PMID: 20014781]
[http://dx.doi.org/10.1021/ol402060q] [PMID: 23915282]
[http://dx.doi.org/10.1021/jo100243c] [PMID: 20232925]
[http://dx.doi.org/10.1021/ol0711117]
[http://dx.doi.org/10.1016/j.tetlet.2005.06.080]
[http://dx.doi.org/10.1039/C9QO00715F]
[http://dx.doi.org/10.1021/acs.orglett.8b00920] [PMID: 29672060]
[http://dx.doi.org/10.1016/j.catcom.2017.06.006]
[http://dx.doi.org/10.1021/ja5116452]
[http://dx.doi.org/10.1039/C7OB00841D] [PMID: 28567460]
[http://dx.doi.org/10.1039/C9OB01749F] [PMID: 31549131]
[http://dx.doi.org/10.1055/s-2007-986665]
[http://dx.doi.org/10.1039/C4RA09153A]
[http://dx.doi.org/10.1039/C7NJ00843K]
[http://dx.doi.org/10.1021/ol101040p] [PMID: 20507088]
[http://dx.doi.org/10.1021/jo3026862] [PMID: 23297649]
[http://dx.doi.org/10.1016/j.tetlet.2019.150967]
[http://dx.doi.org/10.1021/acs.orglett.5b00938] [PMID: 25945598]
[http://dx.doi.org/10.1039/C8OB00999F] [PMID: 30074039]
[http://dx.doi.org/10.1016/j.bmc.2007.03.006] [PMID: 17382550]
[http://dx.doi.org/10.1016/j.tet.2012.03.043]
[http://dx.doi.org/10.1021/acscombsci.7b00170] [PMID: 29381854]
[http://dx.doi.org/10.1002/anie.201300917] [PMID: 23740864]
[http://dx.doi.org/10.1016/j.tetlet.2012.09.026] [PMID: 23139435]
[http://dx.doi.org/10.1002/jccs.201400251]
[http://dx.doi.org/10.3390/molecules16119041] [PMID: 22031067]
[http://dx.doi.org/10.1039/c2ra20662e]
[http://dx.doi.org/10.1021/cc800181y] [PMID: 19245249]
[http://dx.doi.org/10.1039/b003752o]
[http://dx.doi.org/10.1155/2008/739732]
[http://dx.doi.org/10.1002/jhet.267]
[http://dx.doi.org/10.1016/j.tetlet.2015.06.065]
[http://dx.doi.org/10.1055/s-0034-1381135]
[http://dx.doi.org/10.1080/10406638.2019.1632908]
[http://dx.doi.org/10.1016/j.catcom.2009.10.015]
[http://dx.doi.org/10.1016/j.tetlet.2006.07.062]
[http://dx.doi.org/10.21767/2471-9889.100021]
[http://dx.doi.org/10.1002/qsar.200860005]
[http://dx.doi.org/10.1021/cc060109o] [PMID: 17206845]
[http://dx.doi.org/10.3390/molecules21070903] [PMID: 27409599]
[http://dx.doi.org/10.4314/bcse.v32i2.5]
[http://dx.doi.org/10.1016/0040-4039(96)01903-X]
[http://dx.doi.org/10.1039/C4RA06838F]
[http://dx.doi.org/10.17628/ecb.2019.8.405-408]
[http://dx.doi.org/10.1039/C5RA13805A]
[http://dx.doi.org/10.1016/j.tetlet.2010.03.021]
[http://dx.doi.org/10.1016/j.tet.2017.01.035]
[http://dx.doi.org/10.1016/S0040-4039(00)01757-3]
[http://dx.doi.org/10.1016/j.mex.2020.100823] [PMID: 32195140]
[http://dx.doi.org/10.1016/j.tet.2010.11.029]
[http://dx.doi.org/10.1021/acs.joc.8b02019] [PMID: 30246531]
[http://dx.doi.org/10.1039/c4ra04235b]
[http://dx.doi.org/10.1246/cl.190490]